A systems pharmacokinetic/pharmacodynamic model for concizumab to explore the potential of anti-TFPI recycling antibodies
- PMID: 31394258
- PMCID: PMC6824202
- DOI: 10.1016/j.ejps.2019.105032
A systems pharmacokinetic/pharmacodynamic model for concizumab to explore the potential of anti-TFPI recycling antibodies
Abstract
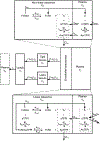
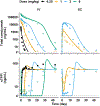
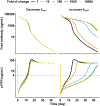
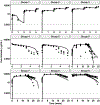
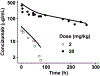
Concizumab is a humanized monoclonal antibody in clinical investigation directed against membrane-bound and soluble tissue factor pathway inhibitor (mTFPI and sTFPI) for treatment of hemophilia. Concizumab displays a non-linear pharmacokinetic (PK) profile due to mTFPI-mediated endocytosis and necessitates a high dose and frequent dosing to suppress the abundant sTFPI, a negative regulator of coagulation. Recycling antibodies that can dissociate bound mTFPI/sTFPI in endosomes for degradation and rescue antibody from degradation have a potential in reducing the dose by extending antibody systemic persistence and sTFPI suppression. We developed a systems PK/pharmacodynamics (PD) model with nested endosome compartments to simulate the effect of decreased antibody binding to mTFPI/sTFPI in endosomes on antibody clearance and sTFPI suppression for exploring the potential of anti-TFPI recycling antibodies in reducing the dose. A dynamic model-building strategy was taken. A reduced PK/PD model without the endosome compartments was developed to optimize unknown target turnover parameters using concizumab PK data. The optimized parameters were then employed in the systems PK/PD model for simulations. The obtained systems PK/PD model adequately described the PK of concizumab in rabbits, monkeys, and humans and the PD in humans. The systems PK/PD model predicted that an anti-TFPI recycling antibody with a 100-fold higher mTFPI/sTFPI dissociation constant in endosomes than concizumab can extend sTFPI suppression from 12 days to 1 month. Thus, the systems PK/PD model provides a quantitative platform for guiding the engineering and translational development of anti-TFPI recycling antibodies.
Keywords: Concizumab; FcRn; Pharmacokinetics modeling; Recycling antibody; Target-mediated drug disposition; Tissue factor pathway inhibitor.
Copyright © 2019 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declarations of interest
F. Rode was an employee of Novo Nordisk, Denmark and is an employee of H. Lundbeck A/S. Concizumab is an investigational drug of Novo Nordisk.
Figures
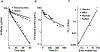

References
-
- Agerso H, Overgaard RV, Petersen MB, Hansen L, Hermit MB, Sorensen MH, Petersen LC, Hilden I, 2014. Pharmacokinetics of an anti-TFPI monoclonal antibody (concizumab) blocking the TFPI interaction with the active site of FXa in Cynomolgus monkeys after iv and sc administration. Eur J Pharm Sci 56, 65–69. - PubMed
-
- al, A.S.e.m., 2018. DescTools: Tools for descriptive statistics. R package version 0.99.24.
-
- Betts A, Keunecke A, van Steeg TJ, van der Graaf PH, Avery LB, Jones H, Berkhout J, 2018. Linear pharmacokinetic parameters for monoclonal antibodies are similar within a species and across different pharmacological targets: A comparison between human, cynomolgus monkey and hFcRn Tg32 transgenic mouse using a population-modeling approach. MAbs 10, 751–764. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

