Macrophages Educated with Exosomes from Primed Mesenchymal Stem Cells Treat Acute Radiation Syndrome by Promoting Hematopoietic Recovery
- PMID: 31394269
- PMCID: PMC6861683
- DOI: 10.1016/j.bbmt.2019.07.026
Macrophages Educated with Exosomes from Primed Mesenchymal Stem Cells Treat Acute Radiation Syndrome by Promoting Hematopoietic Recovery
Abstract
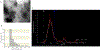
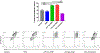
In the setting of radiation-induced trauma, exposure to high levels of radiation can cause an acute radiation syndrome (ARS) causing bone marrow (BM) failure, leading to life-threatening infections, anemia, and thrombocytopenia. We have previously shown that human macrophages educated with human mesenchymal stem cells (MSCs) by coculture can significantly enhance survival of mice exposed to lethal irradiation. In this study, we investigated whether exosomes isolated from MSCs could replace direct coculture with MSCs to generate exosome educated macrophages (EEMs). Functionally unique phenotypes were observed by educating macrophages with exosomes from MSCs (EEMs) primed with bacterial lipopolysaccharide (LPS) at different concentrations (LPS-low EEMs or LPS-high EEMs). LPS-high EEMs were significantly more effective than uneducated macrophages, MSCs, EEMs, or LPS-low EEMs in extending survival after lethal ARS in vivo. Moreover, LPS-high EEMs significantly reduced clinical signs of radiation injury and restored hematopoietic tissue in the BM and spleen as determined by complete blood counts and histology. LPS-high EEMs showed significant increases in gene expression of STAT3, secretion of cytokines like IL-10 and IL-15, and production of growth factors like FLT-3L. LPS-EEMs also showed increased phagocytic activity, which may aid with tissue remodeling. LPS-high EEMs have the potential to be an effective cellular therapy for the management of ARS.
Keywords: Acute radiation syndrome; Exosomes; Hematopoiesis; Macrophages; Mesenchymal stem cells; Radiation injury.
Copyright © 2019 American Society for Transplantation and Cellular Therapy. Published by Elsevier Inc. All rights reserved.
Figures




References
-
- Singh VK, Seed TM. A review of radiation countermeasures focusing on injury-specific medicinals and regulatory approval status: part I. Radiation sub-syndromes, animal models and FDA-approved countermeasures. Int J Radiat Biol. September 2017;93(9):851–869. - PubMed
-
- Singh VK, Garcia M, Seed TM. A review of radiation countermeasures focusing on injury-specific medicinals and regulatory approval status: part II. Countermeasures for limited indications, internalized radionuclides, emesis, late effects, and agents demonstrating efficacy in large animals with or without FDA IND status. Int J Radiat Biol. September 2017;93(9):870–884. - PubMed
-
- Singh VK, Newman VL, Seed TM. Colony-stimulating factors for the treatment of the hematopoietic component of the acute radiation syndrome (H-ARS): a review. Cytokine. January 2015;71(1):22–37. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

