Current Status in Testing for Nonalcoholic Fatty Liver Disease (NAFLD) and Nonalcoholic Steatohepatitis (NASH)
- PMID: 31394730
- PMCID: PMC6721710
- DOI: 10.3390/cells8080845
Current Status in Testing for Nonalcoholic Fatty Liver Disease (NAFLD) and Nonalcoholic Steatohepatitis (NASH)
Abstract
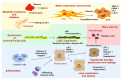
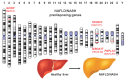
Nonalcoholic fatty liver disease (NAFLD) is the most common chronic liver disease in Western countries with almost 25% affected adults worldwide. The growing public health burden is getting evident when considering that NAFLD-related liver transplantations are predicted to almost double within the next 20 years. Typically, hepatic alterations start with simple steatosis, which easily progresses to more advanced stages such as nonalcoholic steatohepatitis (NASH), fibrosis and cirrhosis. This course of disease finally leads to end-stage liver disease such as hepatocellular carcinoma, which is associated with increased morbidity and mortality. Although clinical trials show promising results, there is actually no pharmacological agent approved to treat NASH. Another important problem associated with NASH is that presently the liver biopsy is still the gold standard in diagnosis and for disease staging and grading. Because of its invasiveness, this technique is not well accepted by patients and the method is prone to sampling error. Therefore, an urgent need exists to find reliable, accurate and noninvasive biomarkers discriminating between different disease stages or to develop innovative imaging techniques to quantify steatosis.
Keywords: algorithms; biomarkers; fibrosis; grading; imaging; nonalcoholic steatohepatitis; scores; staging.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

