High Level Expression of MHC-II in HPV+ Head and Neck Cancers Suggests that Tumor Epithelial Cells Serve an Important Role as Accessory Antigen Presenting Cells
- PMID: 31394808
- PMCID: PMC6721589
- DOI: 10.3390/cancers11081129
High Level Expression of MHC-II in HPV+ Head and Neck Cancers Suggests that Tumor Epithelial Cells Serve an Important Role as Accessory Antigen Presenting Cells
Abstract
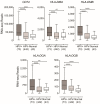
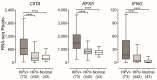
High-risk human papillomaviruses (HPVs) are responsible for a subset of head and neck squamous cell carcinomas (HNSCC). Expression of class II major histocompatibility complex (MHC-II) is associated with antigen presenting cells (APCs). During inflammation, epithelial cells can be induced to express MHC-II and function as accessory APCs. Utilizing RNA-seq data from over 500 HNSCC patients from The Cancer Genome Atlas, we determined the impact of HPV-status on the expression of MHC-II genes and related genes involved in their regulation, antigen presentation, and T-cell co-stimulation. Expression of virtually all MHC-II genes was significantly upregulated in HPV+ carcinomas compared to HPV- or normal control tissue. Similarly, genes that encode products involved in antigen presentation were also significantly upregulated in the HPV+ cohort. In addition, the expression of CIITA and RFX5-regulators of MHC-II-were significantly upregulated in HPV+ tumors. This coordinated upregulation of MHC-II genes was correlated with higher intratumoral levels of interferon-gamma in HPV+ carcinomas. Furthermore, genes that encode various co-stimulatory molecules involved in T-cell activation and survival were also significantly upregulated in HPV+ tumors. Collectively, these results suggest a previously unappreciated role for epithelial cells in antigen presentation that functionally contributes to the highly immunogenic tumor microenvironment observed in HPV+ HNSCC.
Keywords: MHC-II; T-cell; antigen presentation; co-stimulatory molecules; head and neck carcinoma; human papillomavirus; major histocompatibility complex; survival signals.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Gillison M.L., Koch W.M., Capone R.B., Spafford M., Westra W.H., Wu L., Zahurak M.L., Daniel R.W., Viglione M., Symer D.E., et al. Evidence for a Causal Association between Human Papillomavirus and a Subset of Head and Neck Cancers. J. Natl. Cancer Inst. 2000;92:709–720. doi: 10.1093/jnci/92.9.709. - DOI - PubMed
-
- Syrjänen S. Human Papillomavirus (HPV) in Head and Neck Cancer. J. Clin. Virol. 2005;32(Suppl. 1):S59–S66. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

