A major role of TWEAK/Fn14 axis as a therapeutic target for post-angioplasty restenosis
- PMID: 31395500
- PMCID: PMC6712059
- DOI: 10.1016/j.ebiom.2019.07.072
A major role of TWEAK/Fn14 axis as a therapeutic target for post-angioplasty restenosis
Abstract
Background: Tumor necrosis factor-like weak inducer of apoptosis (Tnfsf12; TWEAK) and its receptor Fibroblast growth factor-inducible 14 (Tnfrsf12a; Fn14) participate in the inflammatory response associated with vascular remodeling. However, the functional effect of TWEAK on vascular smooth muscle cells (VSMCs) is not completely elucidated.
Methods: Next generation sequencing-based methods were performed to identify genes and pathways regulated by TWEAK in VSMCs. Flow-citometry, wound-healing scratch experiments and transwell migration assays were used to analyze VSMCs proliferation and migration. Mouse wire injury model was done to evaluate the role of TWEAK/Fn14 during neointimal hyperplasia.
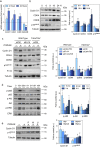
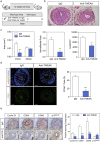
Findings: TWEAK up-regulated 1611 and down-regulated 1091 genes in VSMCs. Using a gene-set enrichment method, we found a functional module involved in cell proliferation defined as the minimal network connecting top TWEAK up-regulated genes. In vitro experiments in wild-type or Tnfrsf12a deficient VSMCs demonstrated that TWEAK increased cell proliferation, VSMCs motility and migration. Mechanistically, TWEAK increased cyclins (cyclinD1), cyclin-dependent kinases (CDK4, CDK6) and decreased cyclin-dependent kinase inhibitors (p15lNK4B) mRNA and protein expression. Downregulation of p15INK4B induced by TWEAK was mediated by mitogen-activated protein kinase ERK and Akt activation. Tnfrsf12a or Tnfsf12 genetic depletion and pharmacological intervention with TWEAK blocking antibody reduced neointimal formation, decreasing cell proliferation, cyclin D1 and CDK4/6 expression, and increasing p15INK4B expression compared with wild type or IgG-treated mice in wire-injured femoral arteries. Finally, immunohistochemistry in human coronary arteries with stenosis or in-stent restenosis revealed high levels of Fn14, TWEAK and PCNA in VSMCs enriched areas of the neointima as compared with healthy coronary arteries.
Interpretation: Our data define a major role of TWEAK/Fn14 in the control of VSMCs proliferation and migration during neointimal hyperplasia after wire injury in mice, and identify TWEAK/Fn14 as a potential target for treating in-stent restenosis. FUND: ISCiii-FEDER, CIBERCV and CIBERDEM.
Keywords: Cyclins; Fn14; Proliferation; Restenosis; TWEAK.
Copyright © 2019. Published by Elsevier B.V.
Conflict of interest statement
None.
Figures







References
-
- Dzau V.J., Braun-Dullaeus R.C., Sedding D.G. Vascular proliferation and atherosclerosis: new perspectives and therapeutic strategies. Nat Med. 2002;8:1249–1256. - PubMed
-
- Alexander M.R., Owens G.K. Epigenetic control of smooth muscle cell differentiation and phenotypic switching in vascular development and disease. Annu Rev Physiol. 2012;74:13–40. - PubMed
-
- Chaabane C., Otsuka F., Virmani R., Bochaton-Piallat M.-L. Biological responses in stented arteries. Cardiovasc Res. 2013;99:353–363. - PubMed
-
- Iakovou I., Schmidt T., Bonizzoni E., Ge L., Sangiorgi G.M., Stankovic G. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA. 2005;293:2126–2130. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

