Fabrication of Bimetal CuFe2O4 Oxide Redox-Active Nanocatalyst for Oxidation of Pinene to Renewable Aroma Oxygenates
- PMID: 31395824
- PMCID: PMC6723823
- DOI: 10.3390/nano9081140
Fabrication of Bimetal CuFe2O4 Oxide Redox-Active Nanocatalyst for Oxidation of Pinene to Renewable Aroma Oxygenates
Abstract
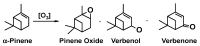
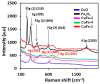
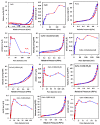
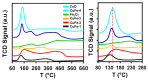
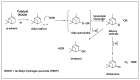
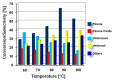
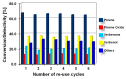
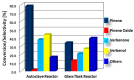
This study report on the synthesis of spinel CuFe2O4 nanostructures by surfactant-assisted method. The catalysts were characterized by X-ray diffraction (XRD), laser Raman, transition electron microscope (TEM), scanning electron microscope (SEM), energy dispersive X-ray (EDX), hydrogen temperature programmed reduction (H2-TPR), and Brunauer-Teller-Emmett-Teller (BET) surface area techniques. CuFe2O4 was active for pinene oxidation using tertiary butyl hydroperoxide (TBHP) to pinene oxide, verbenol, and verbenone aroma oxygenates. Under optimized reaction conditions, the spinel CuFe2O4 catalyst could afford 80% pinene conversion at a combined verbenol/verbenone selectivity of 76% within the reaction time of 20 h. The changes in catalyst synthesis solvent composition ratios induced significantly varying redox, phases, and textural structure features, which resulted in various catalytic enhancement effect. Characterization results showed the spinel CuFe2O4 catalyst possessing less than 5 wt% impurity phases, Cu(OH)2, and CuO to afford the best catalytic performance. The CuFe2O4 catalyst was recyclable to up to five reaction cycles without loss of its activity. The recyclability of the bimetal CuFe2O4 oxide catalyst was simply rendered by use of an external magnet to separate it from the liquid solution.
Keywords: biomass; copper oxide; iron oxide; nanoparticles; pinene; selective oxidation.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures



References
-
- Monteiro J.L.F., Veloso C.O. Catalytic conversion of terpenes into fine chemicals. Top. Catal. 2004;27:169–180. doi: 10.1023/B:TOCA.0000013551.99872.8d. - DOI
-
- Schwab W., Fuchs C., Huang F.-C. Transformation of terpenes into fine chemicals. Eur. J. Lipid Sci. Technol. 2013;115:3–8. doi: 10.1002/ejlt.201200157. - DOI
-
- Mäki-Arvela P., Holmbom B., Salmi T., Murzin D.Y. Recent progress in synthesis of fine and specialty chemicals from wood and other biomass by heterogeneous catalytic processes. Catal. Rev. 2007;49:197–340. doi: 10.1080/01614940701313127. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

