The droplet size of emulsion adjuvants has significant impact on their potency, due to differences in immune cell-recruitment and -activation
- PMID: 31395915
- PMCID: PMC6687744
- DOI: 10.1038/s41598-019-47885-z
The droplet size of emulsion adjuvants has significant impact on their potency, due to differences in immune cell-recruitment and -activation
Abstract
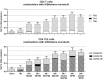
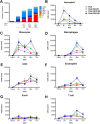
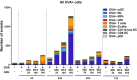
Self-emulsification is routinely used for oral delivery of lipophilic drugs in vivo, with the emulsion forming in vivo. We modified this technique to prepare novel oil-in-water emulsions of varying droplet size and composition on bench to enable adjuvanted vaccine delivery. We used these formulations to show that smaller droplets (20 nm) were much less effective as adjuvants for an influenza vaccine in mice than the emulsion droplet size of commercial influenza vaccine adjuvants (~160 nm). This was unexpected, given the many claims in the literature of the advantages of smaller particulates. We also undertook cell-recruitment mechanistic studies at site of injection and draining lymph nodes to directly address the question of why the smaller droplets were less effective. We discovered that emulsion droplet size and composition have a considerable impact on the ability to recruit immune cells to the injection site. We believe that further work is warranted to more extensively explore the question of whether, the smaller is not 'better', is a more common observation for particulate adjuvants.
Conflict of interest statement
This study was sponsored by Novartis Vaccines & Diagnostics Srl; in March 2015 the Novartis non-influenza Vaccines business was acquired by the GSK group of companies. The sponsor was involved in all stages of the study conduct and analysis. During the time of this study, R. Shah was a student of the Northeastern University under a PhD fellowship from Novartis, and was then an employee of the GSK group of companies from September to December 2015. L. Brito was, A. Seubert, M. Taccone, E. Monaci, A. Bonci and D. O’Hagan are employees of the GSK group of companies. D. O’Hagan and A. Seubert report shares in GSK. Ruchi Shah, Luis Brito, Mansoor Amiji and Derek O’Hagan are listed as inventors on patents on SEA owned by the GSK group of companies.
Figures

References
-
- Shah, R. R., Brito, L. A., O’Hagan, D. T. & Amiji, M. M. In Subunit Vaccine Delivery (eds Camilla Foged, Thomas Rades, Yvonne Perrie, & Sarah Hook) 59–76 (Springer New York, 2015).
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

