Developmental Regulation of KCC2 Phosphorylation Has Long-Term Impacts on Cognitive Function
- PMID: 31396048
- PMCID: PMC6664008
- DOI: 10.3389/fnmol.2019.00173
Developmental Regulation of KCC2 Phosphorylation Has Long-Term Impacts on Cognitive Function
Abstract
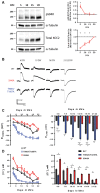
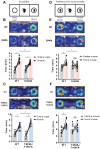
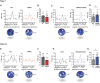
GABAA receptor-mediated currents shift from excitatory to inhibitory during postnatal brain development in rodents. A postnatal increase in KCC2 protein expression is considered to be the sole mechanism controlling the developmental onset of hyperpolarizing synaptic transmission, but here we identify a key role for KCC2 phosphorylation in the developmental EGABA shift. Preventing phosphorylation of KCC2 in vivo at either residue serine 940 (S940), or at residues threonine 906 and threonine 1007 (T906/T1007), delayed or accelerated the postnatal onset of KCC2 function, respectively. Several models of neurodevelopmental disorders including Rett syndrome, Fragile × and Down's syndrome exhibit delayed postnatal onset of hyperpolarizing GABAergic inhibition, but whether the timing of the onset of hyperpolarizing synaptic inhibition during development plays a role in establishing adulthood cognitive function is unknown; we have used the distinct KCC2-S940A and KCC2-T906A/T1007A knock-in mouse models to address this issue. Altering KCC2 function resulted in long-term abnormalities in social behavior and memory retention. Tight regulation of KCC2 phosphorylation is therefore required for the typical timing of the developmental onset of hyperpolarizing synaptic inhibition, and it plays a fundamental role in the regulation of adulthood cognitive function.
Keywords: KCC2; autism; cognition; depolarizing GABA; memory; phosphorylation; social behavior.
Figures

Comment in
-
Get With the (Developmental) Program.Epilepsy Curr. 2020 Mar 23;20(2):102-104. doi: 10.1177/1535759720901606. eCollection 2020 Mar-Apr. Epilepsy Curr. 2020. PMID: 32313506 Free PMC article. No abstract available.
Similar articles
-
Ionotropic and metabotropic kainate receptor signalling regulates Cl- homeostasis and GABAergic inhibition.J Physiol. 2019 Mar;597(6):1677-1690. doi: 10.1113/JP276901. Epub 2019 Jan 21. J Physiol. 2019. PMID: 30570751 Free PMC article.
-
KCC2 activity is critical in limiting the onset and severity of status epilepticus.Proc Natl Acad Sci U S A. 2015 Mar 17;112(11):3523-8. doi: 10.1073/pnas.1415126112. Epub 2015 Mar 2. Proc Natl Acad Sci U S A. 2015. PMID: 25733865 Free PMC article.
-
WNK1-regulated inhibitory phosphorylation of the KCC2 cotransporter maintains the depolarizing action of GABA in immature neurons.Sci Signal. 2015 Jun 30;8(383):ra65. doi: 10.1126/scisignal.aaa0354. Sci Signal. 2015. PMID: 26126716
-
Two developmental switches in GABAergic signalling: the K+-Cl- cotransporter KCC2 and carbonic anhydrase CAVII.J Physiol. 2005 Jan 1;562(Pt 1):27-36. doi: 10.1113/jphysiol.2004.077495. Epub 2004 Nov 4. J Physiol. 2005. PMID: 15528236 Free PMC article. Review.
-
GABAergic signaling as therapeutic target for autism spectrum disorders.Front Pediatr. 2014 Jul 8;2:70. doi: 10.3389/fped.2014.00070. eCollection 2014. Front Pediatr. 2014. PMID: 25072038 Free PMC article. Review.
Cited by
-
WNKs regulate mouse behavior and alter central nervous system glucose uptake and insulin signaling.bioRxiv [Preprint]. 2024 Jun 22:2024.06.09.598125. doi: 10.1101/2024.06.09.598125. bioRxiv. 2024. PMID: 38915673 Free PMC article. Preprint.
-
Unraveling the socio-cognitive consequences of KCC2 disruption in zebrafish: implications for neurodevelopmental disorders and therapeutic interventions.Front Mol Neurosci. 2024 Oct 14;17:1483238. doi: 10.3389/fnmol.2024.1483238. eCollection 2024. Front Mol Neurosci. 2024. PMID: 39469188 Free PMC article.
-
Association of NKAPL rs1635 With Cognitive Function in Early-Onset Schizophrenia.Front Genet. 2022 Jun 21;13:941171. doi: 10.3389/fgene.2022.941171. eCollection 2022. Front Genet. 2022. PMID: 35801084 Free PMC article.
-
NKCC1 and KCC2: Structural insights into phospho-regulation.Front Mol Neurosci. 2022 Jul 22;15:964488. doi: 10.3389/fnmol.2022.964488. eCollection 2022. Front Mol Neurosci. 2022. PMID: 35935337 Free PMC article. Review.
-
Contribution of Smoothened Receptor Signaling in GABAergic Neurotransmission and Chloride Homeostasis in the Developing Rodent Brain.Front Physiol. 2021 Dec 10;12:798066. doi: 10.3389/fphys.2021.798066. eCollection 2021. Front Physiol. 2021. PMID: 34955901 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

