Bone Marrow Stromal Cells Alleviate Secondary Damage in the Substantia Nigra After Focal Cerebral Infarction in Rats
- PMID: 31396057
- PMCID: PMC6668054
- DOI: 10.3389/fncel.2019.00338
Bone Marrow Stromal Cells Alleviate Secondary Damage in the Substantia Nigra After Focal Cerebral Infarction in Rats
Abstract
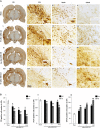
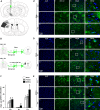
Transplantation of bone marrow stromal cells (BMSCs) is a promising therapy for ischemic stroke. Previously, we had reported that the secondary degeneration occurred in the ipsilateral substantia nigra (SN) after permanent distal branch of middle cerebral artery occlusion (dMCAO) in Sprague-Dawley rats. However, whether BMSCs have neurorestorative effects on the secondary damage in the SN after focal cerebral infarction has not known. In this study, rats were subjected to dMCAO followed by intravenous administration of BMSCs 1 day later. We found that transplanted BMSCs survived and migrated to cortical infarct areas and ipsilateral SN. Furthermore, BMSCs promoted neurogenesis through proliferation and differentiation in the SN after dMCAO. Rats implanted with BMSCs showed significant improvement in their performance of modified neurological severity scores and adhesive-removal test. Engrafted BMSCs enhanced survival of dopaminergic neuron, reduced gliosis in the ipsilateral SN, and increased contents of dopamine (DA) and its metabolites in the ipsilateral striatum after dMCAO. With pseudorabies virus-152 as a retrograde tracer, we also demonstrated that BMSCs could effectively enhance the cortico-striatum-nigral connections. These results suggest that BMSCs transplantation exerts neurorestorative effects after cortical infarction through promoting endogenous neurogenesis, increasing contents of DA and its metabolites, alleviating the secondary neuronal damage in the SN, enhancing the cortico-striatum-nigral projections pathway, and finally improving the neurological functional outcome.
Keywords: bone marrow stromal cells; cerebral infarction; neurorestoration; secondary degeneration; substantia nigra.
Figures




Similar articles
-
Inhibition of Cathepsins B Induces Neuroprotection Against Secondary Degeneration in Ipsilateral Substantia Nigra After Focal Cortical Infarction in Adult Male Rats.Front Aging Neurosci. 2018 May 9;10:125. doi: 10.3389/fnagi.2018.00125. eCollection 2018. Front Aging Neurosci. 2018. PMID: 29867438 Free PMC article.
-
Transplantation of hESCs-Derived Neural Progenitor Cells Alleviates Secondary Damage of Thalamus After Focal Cerebral Infarction in Rats.Stem Cells Transl Med. 2023 Aug 16;12(8):553-568. doi: 10.1093/stcltm/szad037. Stem Cells Transl Med. 2023. PMID: 37399126 Free PMC article.
-
Nogo-A is associated with secondary degeneration of substantia nigra in hypertensive rats with focal cortical infarction.Brain Res. 2012 Aug 21;1469:153-63. doi: 10.1016/j.brainres.2012.06.040. Epub 2012 Jul 4. Brain Res. 2012. PMID: 22771857
-
[Secondary degeneration of the substantia nigra after cerebral infarctions including the striatum].Brain Nerve. 2013 Mar;65(3):289-95. Brain Nerve. 2013. PMID: 23475521 Review. Japanese.
-
Effect of Bone Marrow Stromal Cells in Parkinson's Disease Rodent Model: A Meta-Analysis.Front Aging Neurosci. 2020 Dec 11;12:539933. doi: 10.3389/fnagi.2020.539933. eCollection 2020. Front Aging Neurosci. 2020. PMID: 33362527 Free PMC article.
Cited by
-
Targeting Neurogenesis in Seeking Novel Treatments for Ischemic Stroke.Biomedicines. 2023 Oct 13;11(10):2773. doi: 10.3390/biomedicines11102773. Biomedicines. 2023. PMID: 37893146 Free PMC article. Review.
-
The p75 neurotrophin receptor attenuates secondary thalamic damage after cortical infarction by promoting angiogenesis.CNS Neurosci Ther. 2024 Jul;30(7):e14875. doi: 10.1111/cns.14875. CNS Neurosci Ther. 2024. PMID: 39072998 Free PMC article.
-
MLKL regulates Cx43 ubiquitinational degradation and mediates neuronal necroptosis in ipsilateral thalamus after focal cortical infarction.Mol Brain. 2023 Oct 30;16(1):74. doi: 10.1186/s13041-023-01064-4. Mol Brain. 2023. PMID: 37904209 Free PMC article.
References
LinkOut - more resources
Full Text Sources

