Maturation of Corticospinal Tracts in Children With Hemiplegic Cerebral Palsy Assessed by Diffusion Tensor Imaging and Transcranial Magnetic Stimulation
- PMID: 31396066
- PMCID: PMC6668599
- DOI: 10.3389/fnhum.2019.00254
Maturation of Corticospinal Tracts in Children With Hemiplegic Cerebral Palsy Assessed by Diffusion Tensor Imaging and Transcranial Magnetic Stimulation
Abstract
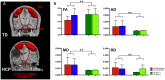
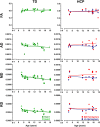
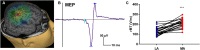
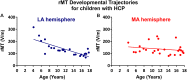
Aim: To assess changes in the developmental trajectory of corticospinal tracts (CST) maturation in children with hemiplegic cerebral palsy (HCP). Methods: Neuroimaging data were obtained from 36 children with HCP for both the more affected (MA) and less affected (LA) hemispheres, and, for purposes of direct comparison, between groups, 15 typically developing (TD) children. With diffusion tensor imaging (DTI), we estimated the mean fractional anisotropy (FA), axial diffusivity (AD), mean diffusivity (MD), and radial diffusivity (RD) of the corticospinal tract, parameters indicative of factors including myelination and axon density. Transcranial magnetic stimulation (TMS) was performed as a neurophysiologic measure of corticospinal tract integrity and organization. Resting motor threshold (rMT) was obtained per hemisphere, per patient. Results: We observed a significant AD and MD developmental trajectory, both of which were inversely related to age (decrease in AD and diffusivity corresponding to increased age) in both hemispheres of TD children (p < 0.001). This maturation process was absent in both MA and LA hemispheres of children with HCP. Additionally, the TMS-derived previously established rMT developmental trajectory was preserved in the LA hemisphere of children with HCP (n = 26; p < 0.0001) but this trajectory was absent in the MA hemisphere. Conclusions: Corticospinal tract maturation arrests in both hemispheres of children with HCP, possibly reflecting perinatal disruption of corticospinal tract myelination and axonal integrity.
Keywords: corticospinal tracts; development; hemiplegic cerebral palsy; maturation; transcranial magnetic stimulation.
Figures
Similar articles
-
Reorganization of the somatosensory cortex in hemiplegic cerebral palsy associated with impaired sensory tracts.Neuroimage Clin. 2017 Oct 19;17:198-212. doi: 10.1016/j.nicl.2017.10.021. eCollection 2018. Neuroimage Clin. 2017. PMID: 29159037 Free PMC article.
-
Differential involvement of corticospinal tract (CST) fibers in UMN-predominant ALS patients with or without CST hyperintensity: A diffusion tensor tractography study.Neuroimage Clin. 2017 Feb 22;14:574-579. doi: 10.1016/j.nicl.2017.02.017. eCollection 2017. Neuroimage Clin. 2017. PMID: 28337412 Free PMC article.
-
Associations between clinical outcome and navigated transcranial magnetic stimulation characteristics in patients with motor-eloquent brain lesions: a combined navigated transcranial magnetic stimulation-diffusion tensor imaging fiber tracking approach.J Neurosurg. 2018 Mar;128(3):800-810. doi: 10.3171/2016.11.JNS162322. Epub 2017 Mar 31. J Neurosurg. 2018. PMID: 28362239
-
Brain functional reorganization in children with hemiplegic cerebral palsy: Assessment with TMS and therapeutic perspectives.Neurophysiol Clin. 2021 Oct;51(5):391-408. doi: 10.1016/j.neucli.2021.09.002. Epub 2021 Oct 4. Neurophysiol Clin. 2021. PMID: 34615605 Review.
-
The role of diffusion tensor imaging and fractional anisotropy in the evaluation of patients with idiopathic normal pressure hydrocephalus: a literature review.Neurosurg Focus. 2016 Sep;41(3):E12. doi: 10.3171/2016.6.FOCUS16192. Neurosurg Focus. 2016. PMID: 27581308 Review.
Cited by
-
Tractography of sensorimotor pathways in dyskinetic cerebral palsy: Association with motor function.Ann Clin Transl Neurol. 2024 Oct;11(10):2609-2622. doi: 10.1002/acn3.52174. Epub 2024 Sep 10. Ann Clin Transl Neurol. 2024. PMID: 39257055 Free PMC article.
-
Changes of Structural Brain Network Following Repetitive Transcranial Magnetic Stimulation in Children With Bilateral Spastic Cerebral Palsy: A Diffusion Tensor Imaging Study.Front Pediatr. 2021 Jan 15;8:617548. doi: 10.3389/fped.2020.617548. eCollection 2020. Front Pediatr. 2021. PMID: 33520901 Free PMC article.
-
The somatosensory cortical activity in individuals with cerebral palsy displays an aberrant developmental trajectory.J Physiol. 2021 Feb;599(4):1281-1289. doi: 10.1113/JP280400. Epub 2020 Dec 22. J Physiol. 2021. PMID: 33296078 Free PMC article.
-
The Challenge of Diffusion Magnetic Resonance Imaging in Cerebral Palsy: A Proposed Method to Identify White Matter Pathways.Brain Sci. 2023 Sep 29;13(10):1386. doi: 10.3390/brainsci13101386. Brain Sci. 2023. PMID: 37891755 Free PMC article.
-
White matter lesions and DTI metrics related to various types of dysfunction in cerebral palsy: A meta-analysis and systematic review.PLoS One. 2025 Jan 24;20(1):e0312378. doi: 10.1371/journal.pone.0312378. eCollection 2025. PLoS One. 2025. PMID: 39854387 Free PMC article.
References
-
- Bender R., Lange S. (2001). Adjusting for multiple testing—when and how? J. Clin. Epidemiol. 54, 343–349. - PubMed
-
- Chiappa K. H., Cros D., Day B., Fang J. J., Macdonell R., Mavroudakis N. (1991). Magnetic stimulation of the human motor cortex: ipsilateral and contralateral facilitation effects. Electroencephalogr. Clin. Neurophysiol. 43, 186–201. - PubMed
-
- Eyre J. A., Miller S. I., Clowry G. J. (2002). “The development of the corticospinal tract in humans,” in Handbook of Transcranial Magnetic Stimulation, eds Pascual-Leone A., Davey G., Rothwell J., Wasserman E. M. (London: Arnold; ), 235–249.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

