The Energy Homeostasis Principle: Neuronal Energy Regulation Drives Local Network Dynamics Generating Behavior
- PMID: 31396067
- PMCID: PMC6664078
- DOI: 10.3389/fncom.2019.00049
The Energy Homeostasis Principle: Neuronal Energy Regulation Drives Local Network Dynamics Generating Behavior
Erratum in
-
Corrigendum: The Energy Homeostasis Principle: Neuronal Energy Regulation Drives Local Network Dynamics Generating Behavior.Front Comput Neurosci. 2020 Oct 29;14:599670. doi: 10.3389/fncom.2020.599670. eCollection 2020. Front Comput Neurosci. 2020. PMID: 33192429 Free PMC article.
Abstract
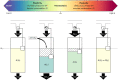
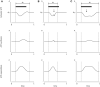
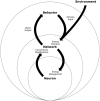
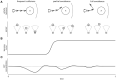
A major goal of neuroscience is understanding how neurons arrange themselves into neural networks that result in behavior. Most theoretical and experimental efforts have focused on a top-down approach which seeks to identify neuronal correlates of behaviors. This has been accomplished by effectively mapping specific behaviors to distinct neural patterns, or by creating computational models that produce a desired behavioral outcome. Nonetheless, these approaches have only implicitly considered the fact that neural tissue, like any other physical system, is subjected to several restrictions and boundaries of operations. Here, we proposed a new, bottom-up conceptual paradigm: The Energy Homeostasis Principle, where the balance between energy income, expenditure, and availability are the key parameters in determining the dynamics of neuronal phenomena found from molecular to behavioral levels. Neurons display high energy consumption relative to other cells, with metabolic consumption of the brain representing 20% of the whole-body oxygen uptake, contrasting with this organ representing only 2% of the body weight. Also, neurons have specialized surrounding tissue providing the necessary energy which, in the case of the brain, is provided by astrocytes. Moreover, and unlike other cell types with high energy demands such as muscle cells, neurons have strict aerobic metabolism. These facts indicate that neurons are highly sensitive to energy limitations, with Gibb's free energy dictating the direction of all cellular metabolic processes. From this activity, the largest energy, by far, is expended by action potentials and post-synaptic potentials; therefore, plasticity can be reinterpreted in terms of their energy context. Consequently, neurons, through their synapses, impose energy demands over post-synaptic neurons in a close loop-manner, modulating the dynamics of local circuits. Subsequently, the energy dynamics end up impacting the homeostatic mechanisms of neuronal networks. Furthermore, local energy management also emerges as a neural population property, where most of the energy expenses are triggered by sensory or other modulatory inputs. Local energy management in neurons may be sufficient to explain the emergence of behavior, enabling the assessment of which properties arise in neural circuits and how. Essentially, the proposal of the Energy Homeostasis Principle is also readily testable for simple neuronal networks.
Keywords: behavior; emergent properties; energy; homeostasis; neuronal networks.
Figures

Similar articles
-
Energy-efficient neural information processing in individual neurons and neuronal networks.J Neurosci Res. 2017 Nov;95(11):2253-2266. doi: 10.1002/jnr.24131. Epub 2017 Aug 22. J Neurosci Res. 2017. PMID: 28833444 Review.
-
The Energy Homeostasis Principle: A Naturalistic Approach to Explain the Emergence of Behavior.Front Syst Neurosci. 2022 Jan 6;15:782781. doi: 10.3389/fnsys.2021.782781. eCollection 2021. Front Syst Neurosci. 2022. PMID: 35069133 Free PMC article.
-
[Dynamic paradigm in psychopathology: "chaos theory", from physics to psychiatry].Encephale. 2001 May-Jun;27(3):260-8. Encephale. 2001. PMID: 11488256 French.
-
Constraints of Metabolic Energy on the Number of Synaptic Connections of Neurons and the Density of Neuronal Networks.Front Comput Neurosci. 2018 Nov 20;12:91. doi: 10.3389/fncom.2018.00091. eCollection 2018. Front Comput Neurosci. 2018. PMID: 30524259 Free PMC article.
-
Maturation of rhythmic neural network: role of central modulatory inputs.J Physiol Paris. 2003 Jan;97(1):59-68. doi: 10.1016/j.jphysparis.2003.10.007. J Physiol Paris. 2003. PMID: 14706691 Review.
Cited by
-
Red Flags in Primary Mitochondrial Diseases: What Should We Recognize?Int J Mol Sci. 2023 Nov 25;24(23):16746. doi: 10.3390/ijms242316746. Int J Mol Sci. 2023. PMID: 38069070 Free PMC article. Review.
-
Metabolic tuning of inhibition regulates hippocampal neurogenesis in the adult brain.Proc Natl Acad Sci U S A. 2020 Oct 13;117(41):25818-25829. doi: 10.1073/pnas.2006138117. Epub 2020 Sep 24. Proc Natl Acad Sci U S A. 2020. PMID: 32973092 Free PMC article.
-
Phase-sensitive detection of anomalous diffusion dynamics in the neuronal membrane induced by ion channel gating.Phys Med Biol. 2023 Mar 13;68(6):065005. doi: 10.1088/1361-6560/acbf9c. Phys Med Biol. 2023. PMID: 36848681 Free PMC article.
-
Autologous mitochondrial transplant for acute cerebral ischemia: Phase 1 trial results and review.J Cereb Blood Flow Metab. 2024 Dec 4:271678X241305230. doi: 10.1177/0271678X241305230. Online ahead of print. J Cereb Blood Flow Metab. 2024. PMID: 39628322 Free PMC article. Review.
-
Single-Cell Radiotracer Allocation via Immunomagnetic Sorting to Disentangle PET Signals at Cellular Resolution.J Nucl Med. 2022 Oct;63(10):1459-1462. doi: 10.2967/jnumed.122.264171. Epub 2022 May 19. J Nucl Med. 2022. PMID: 35589403 Free PMC article.
References
LinkOut - more resources
Full Text Sources

