Method Validation for Simultaneous Quantification of Olmesartan and Hydrochlorothiazide in Human Plasma Using LC-MS/MS and Its Application Through Bioequivalence Study in Healthy Volunteers
- PMID: 31396085
- PMCID: PMC6664239
- DOI: 10.3389/fphar.2019.00810
Method Validation for Simultaneous Quantification of Olmesartan and Hydrochlorothiazide in Human Plasma Using LC-MS/MS and Its Application Through Bioequivalence Study in Healthy Volunteers
Abstract
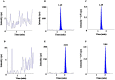
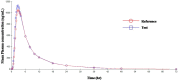
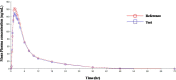
A new, simple, sensitive, selective, rapid, and high-throughput liquid chromatography-tandem mass spectrometry (LC-MS/MS) method has been developed and validated for simultaneous quantification of Olmesartan and hydrochlorothiazide in human plasma. Simple liquid-liquid extraction procedure was applied for plasma sample pretreatment using a mixture of diethyl ether and dichloromethane, as an extraction solution. Analytes were separated on UNISOL C18 150*4.6 mm, 5 µm column using methanol, and 2 mM ammonium acetate pH 5.5 (80:20, v/v) as a mobile phase and detected by electrospray ionization in the multiple reaction monitoring (MRM) mode. The mass transition ion pairs were followed in negative ion mode as m/z 445.20 → 148.90 for Olmesartan; m/z 451.40 → 154.30 for Olmesartan D6 and m/z 295.80 → 205.10 for hydrochlorothiazide; m/z 298.90 → 206.30 for hydrochlorothiazide 13C D2. The method showed excellent linearity (r 2 > 0.99) over the concentration range of 5.002-2,599.934 ng/ml for Olmesartan and from 3.005 to 499.994 ng/ml for hydrochlorothiazide. Precision (% CV) and accuracy (% bias) for Olmesartan were found in the range of 3.07-9.02% and -5.00-0.00%, respectively. Precision (% CV) and accuracy (% bias) for hydrochlorothiazide were found in the range of 3.32-8.21% and 1.99-3.80%, respectively. This as developed novel and high-throughput liquid-liquid extraction bioanalytical method has substantial innovative value with the benefits of cost effectiveness, good extraction efficiency, shorter analysis run time, low organic solvent consumption, and simpler procedure over the previously reported solid-phase extraction method. The application of this method in pharmacokinetic studies was further demonstrated successfully through a bioequivalence study conducted on healthy human subjects, following oral administration of combined formulation of Olmesartan medoxomil and hydrochlorothiazide in fixed-dose tablet.
Keywords: LC-MS/MS; bioequivalence; hydrochlorothiazide; hypertension; olmesartan; pharmacokinetics.
Figures

Similar articles
-
Simultaneous quantification of amiloride and hydrochlorothiazide in human plasma by liquid chromatography-tandem mass spectrometry.J Pharm Anal. 2017 Oct;7(5):288-296. doi: 10.1016/j.jpha.2017.03.007. Epub 2017 Mar 24. J Pharm Anal. 2017. PMID: 29404051 Free PMC article.
-
Fast and sensitive LC-MS/MS method for the simultaneous determination of lisinopril and hydrochlorothiazide in human plasma.J Pharm Anal. 2017 Jun;7(3):163-169. doi: 10.1016/j.jpha.2016.11.004. Epub 2016 Nov 25. J Pharm Anal. 2017. PMID: 29404033 Free PMC article.
-
Simultaneous determination of amiloride and hydrochlorothiazide in human plasma by liquid chromatography/tandem mass spectrometry with positive/negative ion-switching electrospray ionisation.Rapid Commun Mass Spectrom. 2007;21(21):3427-34. doi: 10.1002/rcm.3235. Rapid Commun Mass Spectrom. 2007. PMID: 17902196
-
A liquid chromatography/tandem mass spectrometry method for the simultaneous quantification of valsartan and hydrochlorothiazide in human plasma.J Chromatogr B Analyt Technol Biomed Life Sci. 2007 Jun 1;852(1-2):436-42. doi: 10.1016/j.jchromb.2007.02.014. Epub 2007 Feb 15. J Chromatogr B Analyt Technol Biomed Life Sci. 2007. PMID: 17331816
-
Simultaneous determination of bisoprolol and hydrochlorothiazide in human plasma by HPLC coupled with tandem mass spectrometry.J Chromatogr B Analyt Technol Biomed Life Sci. 2009 Jun 1;877(16-17):1689-97. doi: 10.1016/j.jchromb.2009.04.021. Epub 2009 Apr 18. J Chromatogr B Analyt Technol Biomed Life Sci. 2009. PMID: 19427275
Cited by
-
Comparison of the Determination of Some Antihypertensive Drugs in Clinical Human Plasma Samples by Solvent Front Position Extraction and Precipitation Modes.Molecules. 2023 Feb 27;28(5):2213. doi: 10.3390/molecules28052213. Molecules. 2023. PMID: 36903457 Free PMC article.
-
Geriatric nutrition risk index in the prediction of all-cause and cardiovascular mortality in elderly hypertensive population: NHANES 1999-2016.Front Cardiovasc Med. 2023 Jul 3;10:1203130. doi: 10.3389/fcvm.2023.1203130. eCollection 2023. Front Cardiovasc Med. 2023. PMID: 37465450 Free PMC article.
-
A validated LC-MS/MS method for analysis of Cabergoline in human plasma with its implementation in a bioequivalent study: investigation of method greenness.BMC Chem. 2022 Sep 24;16(1):71. doi: 10.1186/s13065-022-00862-6. BMC Chem. 2022. PMID: 36153557 Free PMC article.
-
A novel method to estimate the absorption rate constant for two-compartment model fitted drugs without intravenous pharmacokinetic data.Front Pharmacol. 2023 May 4;14:1087913. doi: 10.3389/fphar.2023.1087913. eCollection 2023. Front Pharmacol. 2023. PMID: 37214472 Free PMC article.
-
Simultaneous Analysis of a Combination of Anti-Hypertensive Drugs, Fimasartan, Amlodipine, and Hydrochlorothiazide, in Rats Using LC-MS/MS and Subsequent Application to Pharmacokinetic Drug Interaction with Red Ginseng Extract.Toxics. 2022 Sep 30;10(10):576. doi: 10.3390/toxics10100576. Toxics. 2022. PMID: 36287856 Free PMC article.
References
-
- Bramlage P., Zemmrich C., Ketelhut R., Wolf W. P., Fronk E. M., Schmieder R. E. (2013). Safety, tolerability, and efficacy of a fixed dose combination of olmesartan 40 mg and hydrochlorothiazide 12.5/25 mg in daily practice. Vasc. Health Risk Manag. 9, 475–483. 10.2147/VHRM.S49118 - DOI - PMC - PubMed
-
- Doshi N., Seth A., Patel C. N. (2012). Validated reversed phase High Performance Liquid Chromatography method for simultaneous estimation of olmesartan medoxomil, hydrochlorothiazide and amlodipine besylate in newly designed pharmaceutical dosage forms. Int. Res. J. Pharm. 3, 178–182.
LinkOut - more resources
Full Text Sources

