Palmitoylethanolamide (PEA) as a Potential Therapeutic Agent in Alzheimer's Disease
- PMID: 31396087
- PMCID: PMC6667638
- DOI: 10.3389/fphar.2019.00821
Palmitoylethanolamide (PEA) as a Potential Therapeutic Agent in Alzheimer's Disease
Abstract
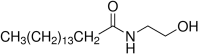
N-Palmitoylethanolamide (PEA) is a non-endocannabinoid lipid mediator belonging to the class of the N-acylethanolamine phospolipids and was firstly isolated from soy lecithin, egg yolk, and peanut meal. Either preclinical or clinical studies indicate that PEA is potentially useful in a wide range of therapeutic areas, including eczema, pain, and neurodegeneration. PEA-containing products are already licensed for use in humans as a nutraceutical, a food supplement, or a food for medical purposes, depending on the country. PEA is especially used in humans for its analgesic and anti-inflammatory properties and has demonstrated high safety and tolerability. Several preclinical in vitro and in vivo studies have proven that PEA can induce its biological effects by acting on several molecular targets in both central and peripheral nervous systems. These multiple mechanisms of action clearly differentiate PEA from classic anti-inflammatory drugs and are attributed to the compound that has quite unique anti(neuro)inflammatory properties. According to this view, preclinical studies indicate that PEA, especially in micronized or ultramicronized forms (i.e., formulations that maximize PEA bioavailability and efficacy), could be a potential therapeutic agent for the effective treatment of different pathologies characterized by neurodegeneration, (neuro)inflammation, and pain. In particular, the potential neuroprotective effects of PEA have been demonstrated in several experimental models of Alzheimer's disease. Interestingly, a single-photon emission computed tomography (SPECT) case study reported that a mild cognitive impairment (MCI) patient, treated for 9 months with ultramicronized-PEA/luteolin, presented an improvement of cognitive performances. In the present review, we summarized the current preclinical and clinical evidence of PEA as a possible therapeutic agent in Alzheimer's disease. The possible PEA neuroprotective mechanism(s) of action is also described.
Keywords: 3xTg-AD; animal models; neuroinflammation; preclinical studies; ultramicronized formulation.
Figures
Similar articles
-
Effects of Palmitoylethanolamide on Neurodegenerative Diseases: A Review from Rodents to Humans.Biomolecules. 2022 May 5;12(5):667. doi: 10.3390/biom12050667. Biomolecules. 2022. PMID: 35625595 Free PMC article. Review.
-
Chronic Oral Palmitoylethanolamide Administration Rescues Cognitive Deficit and Reduces Neuroinflammation, Oxidative Stress, and Glutamate Levels in A Transgenic Murine Model of Alzheimer's Disease.J Clin Med. 2020 Feb 5;9(2):428. doi: 10.3390/jcm9020428. J Clin Med. 2020. PMID: 32033363 Free PMC article.
-
Ultramicronized N-palmitoylethanolamine associated with analgesics: Effects against persistent pain.Pharmacol Ther. 2024 Jun;258:108649. doi: 10.1016/j.pharmthera.2024.108649. Epub 2024 Apr 12. Pharmacol Ther. 2024. PMID: 38615798 Review.
-
An Update of Palmitoylethanolamide and Luteolin Effects in Preclinical and Clinical Studies of Neuroinflammatory Events.Antioxidants (Basel). 2020 Mar 5;9(3):216. doi: 10.3390/antiox9030216. Antioxidants (Basel). 2020. PMID: 32150935 Free PMC article. Review.
-
Chronic palmitoylethanolamide administration via slow-release subcutaneous pellets promotes neuroprotection and mitigates neuroinflammation in the Tg2576 mouse model of Alzheimer's disease.Front Cell Neurosci. 2025 Apr 17;19:1571428. doi: 10.3389/fncel.2025.1571428. eCollection 2025. Front Cell Neurosci. 2025. PMID: 40313591 Free PMC article.
Cited by
-
Fatty acid amide hydrolase activity in the dorsal periaqueductal gray attenuates neuropathic pain and associated dysautonomia.Am J Physiol Regul Integr Comp Physiol. 2022 Nov 1;323(5):R749-R762. doi: 10.1152/ajpregu.00073.2022. Epub 2022 Sep 26. Am J Physiol Regul Integr Comp Physiol. 2022. PMID: 36154489 Free PMC article.
-
ALIAmides Update: Palmitoylethanolamide and Its Formulations on Management of Peripheral Neuropathic Pain.Int J Mol Sci. 2020 Jul 27;21(15):5330. doi: 10.3390/ijms21155330. Int J Mol Sci. 2020. PMID: 32727084 Free PMC article. Review.
-
Palmitoylethanolamide and White Matter Lesions: Evidence for Therapeutic Implications.Biomolecules. 2022 Aug 27;12(9):1191. doi: 10.3390/biom12091191. Biomolecules. 2022. PMID: 36139030 Free PMC article. Review.
-
A Decades-Long Journey of Palmitoylethanolamide (PEA) for Chronic Neuropathic Pain Management: A Comprehensive Narrative Review.Pain Ther. 2025 Feb;14(1):81-101. doi: 10.1007/s40122-024-00685-4. Epub 2024 Dec 4. Pain Ther. 2025. PMID: 39630391 Free PMC article. Review.
-
Effects of Palmitoylethanolamide on Neurodegenerative Diseases: A Review from Rodents to Humans.Biomolecules. 2022 May 5;12(5):667. doi: 10.3390/biom12050667. Biomolecules. 2022. PMID: 35625595 Free PMC article. Review.
References
-
- Artamonov M., Zhukov O., Shuba I., Storozhuk L., Khmel T., Klimashevsky V., et al. (2005). Incorporation of labelled N-acylethanolamine (NAE) into rat brain regions in vivo and adaptive properties of saturated NAE under X-ray irradiation. Ukr. Biokhim. Zh. 77(6), 51–62. - PubMed
-
- Avagliano C., Russo R., De Caro C., Cristiano C., La Rana G., Piegari G., et al. (2016). Palmitoylethanolamide protects mice against 6-OHDA-induced neurotoxicity and endoplasmic reticulum stress: in vivo and in vitro evidence. Pharmacol. Res. 113 (Pt A), 276–289. 10.1016/j.phrs.2016.09.004 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources