Effects of Molecular Hydrogen on Methamphetamine-Induced Neurotoxicity and Spatial Memory Impairment
- PMID: 31396089
- PMCID: PMC6664236
- DOI: 10.3389/fphar.2019.00823
Effects of Molecular Hydrogen on Methamphetamine-Induced Neurotoxicity and Spatial Memory Impairment
Abstract
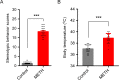
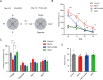
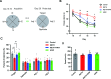
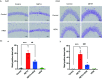
Methamphetamine (METH) is a highly addictive stimulant, and METH exposure can induce irreversible neuronal damage and cause neuropsychiatric and cognitive disorders. The ever-increasing levels of METH abuse worldwide have necessitated the identification of effective intervention strategies to protect the brain against METH-induced neurotoxicity. The protective effects of molecular hydrogen on oxidative stress and related neurodegenerative diseases have been recently elucidated. Herein, we investigated whether treatment with molecular hydrogen ameliorated the METH-induced neurotoxicity and spatial learning and memory impairments. Male C57BL/6 mice received four intraperitoneal METH injections (10 mg/kg, 3-h interval), and stereotypic behaviors and hyperthermia were observed. After METH treatment and behavioral observation, the mice were returned to their home cages, where they received water or hydrogen-rich water (HRW) ad libitum for 7 days. We found that the molecular hydrogen delivered by ad libitum HRW consumption significantly inhibited the METH-induced spatial learning impairment and memory loss evidenced in the Barnes maze and Morris water maze tests. Furthermore, molecular hydrogen significantly restrained the neuronal damage in the hippocampus after high-dose METH exposure. Ad libitum HRW consumption also had an inhibitory effect on the METH-induced increase in the expression of Bax/Bcl-2, cleaved caspase-3, glucose-related protein 78 (GRP 78), CCAAT/enhancer-binding protein homologous protein (CHOP), and p-NF-kB p65 expression and elevation of interleukin (IL)-6 and tumor necrosis factor (TNF)-α levels in the hippocampus. These are the first findings to indicate that hydrogen might ameliorate METH-induced neurotoxicity and has a potential application in reducing the risk of neurodegeneration frequently observed in METH abusers.
Keywords: endoplasmic reticulum stress; methamphetamine; mitochondrial dysfunction; molecular hydrogen; neuroinflammation; spatial learning and memory impairment.
Figures

Similar articles
-
The Role of Hyperthermia in Methamphetamine-Induced Depression-Like Behaviors: Protective Effects of Coral Calcium Hydride.Front Mol Neurosci. 2022 Jan 4;14:808807. doi: 10.3389/fnmol.2021.808807. eCollection 2021. Front Mol Neurosci. 2022. PMID: 35058751 Free PMC article.
-
Erythropoietin improve spatial memory impairment following methamphetamine neurotoxicity by inhibition of apoptosis, oxidative stress and neuroinflammation in CA1 area of hippocampus.J Chem Neuroanat. 2022 Oct;124:102137. doi: 10.1016/j.jchemneu.2022.102137. Epub 2022 Jul 14. J Chem Neuroanat. 2022. PMID: 35842017
-
Hydrogen Sulfide Protects Hippocampal Neurons Against Methamphetamine Neurotoxicity Via Inhibition of Apoptosis and Neuroinflammation.J Mol Neurosci. 2019 Jan;67(1):133-141. doi: 10.1007/s12031-018-1218-8. Epub 2018 Nov 19. J Mol Neurosci. 2019. PMID: 30456731
-
Methamphetamine-Induced Neuronal Damage: Neurotoxicity and Neuroinflammation.Biomol Ther (Seoul). 2020 Sep 1;28(5):381-388. doi: 10.4062/biomolther.2020.044. Biomol Ther (Seoul). 2020. PMID: 32668144 Free PMC article. Review.
-
Detection of methamphetamine neurotoxicity in forensic autopsy cases.Leg Med (Tokyo). 2009 Apr;11 Suppl 1:S63-5. doi: 10.1016/j.legalmed.2009.01.003. Epub 2009 Mar 6. Leg Med (Tokyo). 2009. PMID: 19269222 Review.
Cited by
-
Bi-enzymes treatments attenuate cognitive impairment associated with oxidative damage of heavy metals.R Soc Open Sci. 2021 Jan 13;8(1):201404. doi: 10.1098/rsos.201404. eCollection 2021 Jan. R Soc Open Sci. 2021. PMID: 33614079 Free PMC article.
-
Low and high dose methamphetamine differentially regulate synaptic structural plasticity in cortex and hippocampus.Front Cell Neurosci. 2022 Nov 2;16:1003617. doi: 10.3389/fncel.2022.1003617. eCollection 2022. Front Cell Neurosci. 2022. PMID: 36406748 Free PMC article.
-
The Role of Hyperthermia in Methamphetamine-Induced Depression-Like Behaviors: Protective Effects of Coral Calcium Hydride.Front Mol Neurosci. 2022 Jan 4;14:808807. doi: 10.3389/fnmol.2021.808807. eCollection 2021. Front Mol Neurosci. 2022. PMID: 35058751 Free PMC article.
-
LY-2183240 enhances reward-seeking behavior with inducing neuronal excitation and early apoptosis in mouse.iScience. 2024 Sep 30;27(11):111069. doi: 10.1016/j.isci.2024.111069. eCollection 2024 Nov 15. iScience. 2024. PMID: 39524361 Free PMC article.
-
Therapeutic Effects of Hydrogen Gas Inhalation on Trimethyltin-Induced Neurotoxicity and Cognitive Impairment in the C57BL/6 Mice Model.Int J Mol Sci. 2021 Dec 10;22(24):13313. doi: 10.3390/ijms222413313. Int J Mol Sci. 2021. PMID: 34948107 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials

