Heme Catabolic Pathway in Inflammation and Immune Disorders
- PMID: 31396090
- PMCID: PMC6667928
- DOI: 10.3389/fphar.2019.00825
Heme Catabolic Pathway in Inflammation and Immune Disorders
Abstract
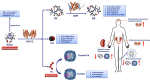
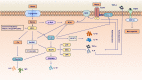
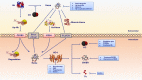
In recent years, the heme catabolic pathway is considered to play an important regulatory role in cell protection, apoptosis, inflammation, and other physiological and pathological processes. An appropriate amount of heme forms the basic elements of various life activities, while when released in large quantities, it can induce toxicity by mediating oxidative stress and inflammation. Heme oxygenase (HO) -1 can catabolize free heme into carbon monoxide (CO), ferrous iron, and biliverdin (BV)/bilirubin (BR). The diverse functions of these metabolites in immune systems are fascinating. Decades work shows that administration of degradation products of heme such as CO and BV/BR exerts protective activities in systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), multiple sclerosis (MS) and other immune disorders. This review elaborates the molecular and biochemical characterization of heme catabolic pathway, discusses the signal transduction and immunomodulatory mechanism in inflammation and summarizes the promising therapeutic strategies based on this pathway in inflammatory and immune disorders.
Keywords: biliverdin; carbon monoxide; heme; heme oxygenase; immune disorders; inflammation.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources

