Site-Specific DC Surface Signatures Influence CD4+ T Cell Co-stimulation and Lung-Homing
- PMID: 31396211
- PMCID: PMC6668556
- DOI: 10.3389/fimmu.2019.01650
Site-Specific DC Surface Signatures Influence CD4+ T Cell Co-stimulation and Lung-Homing
Erratum in
-
Corrigendum: Site-Specific DC Surface Signatures Influence CD4+ T Cell Co-stimulation and Lung-Homing.Front Immunol. 2019 Nov 11;10:2640. doi: 10.3389/fimmu.2019.02640. eCollection 2019. Front Immunol. 2019. PMID: 31777477 Free PMC article.
Abstract
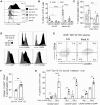
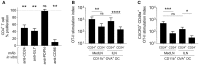
Dendritic cells (DCs) that drain the gut and skin are known to favor the establishment of T cell populations that home to the original site of DC-antigen (Ag) encounter by providing soluble "imprinting" signals to T cells in the lymph node (LN). To study the induction of lung T cell-trafficking, we used a protein-adjuvant murine intranasal and intramuscular immunization model to compare in vivo-activated Ag+ DCs in the lung and muscle-draining LNs. Higher frequencies of Ag+ CD11b+ DCs were observed in lung-draining mediastinal LNs (MedLN) compared to muscle-draining inguinal LNs (ILN). Ag+ CD11b+ MedLN DCs were qualitatively superior at priming CD4+ T cells, which then expressed CD49a and CXCR3, and preferentially trafficked into the lung parenchyma. CD11b+ DCs from the MedLN expressed higher levels of surface podoplanin, Trem4, GL7, and the known co-stimulatory molecules CD80, CD86, and CD24. Blockade of specific MedLN DC molecules or the use of sorted DC and T cell co-cultures demonstrated that DC surface phenotype influences the ability to prime T cells that then home to the lung. Thus, the density of dLN Ag+ DCs, and DC surface molecule signatures are factors that can influence the output and differentiation of lung-homing CD4+ T cells.
Keywords: CD11b+ dendritic cells; costimulation; lung CD4+ T cells; lung homing; tissue imprinting; vaccination route.
Figures




References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

