The antimicrobial peptides and their potential clinical applications
- PMID: 31396309
- PMCID: PMC6684887
The antimicrobial peptides and their potential clinical applications
Abstract
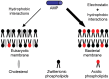
Nowadays, the bacterial drug resistance leads to serious healthy problem worldwide due to the long-term use and the abuse of traditional antibiotics result in drug resistance of bacteria. Finding a new antibiotic is becoming more and more difficult. Antimicrobial peptides (AMPs) are the host defense peptides with most of them being the cationic (positively charged) and amphiphilic (hydrophilic and hydrophobic) α-helical peptide molecules. The membrane permeability is mostly recognized as the well-accepted mechanism to describe the action of cationic AMPs. These cationic AMPs can bind and interact with the negatively charged bacterial cell membranes, leading to the change of the electrochemical potential on bacterial cell membranes, inducing cell membrane damage and the permeation of larger molecules such as proteins, destroying cell morphology and membranes and eventually resulting in cell death. These AMPs have been demonstrated to have their own advantages over the traditional antibiotics with a broad-spectrum of antimicrobial activities including anti-bacteria, anti-fungi, anti-viruses, and anti-cancers, and even overcome bacterial drug-resistance. The natural AMPs exist in a variety of organisms and are not stable with a short half-life, more or less toxic side effects, and particularly may have severe hemolytic activity. To open the clinical applications, it is necessary and important to develop the synthetic and long-lasting AMP analogs that overcome the disadvantages of their natural peptides and the potential problems for the drug candidates.
Keywords: Antimicrobial peptides; amphiphilic; antibiotics; cationic; hydrophilic; hydrophobic; membrane permeability; microbes.
Conflict of interest statement
None.
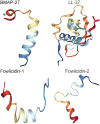
Figures



References
-
- Moravej H, Moravej Z, Yazdanparast M, Heiat M, Mirhosseini A, Moosazadeh Moghaddam M, Mirnejad R. Antimicrobial peptides: features, action, and their resistance mechanisms in bacteria. Microb Drug Resist. 2018;24:747–67. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
