Evaluation of the 5-fluorouracil plasma level in patients with colorectal cancer undergoing continuous infusion chemotherapy
- PMID: 31396387
- PMCID: PMC6667885
- DOI: 10.3892/mco.2019.1893
Evaluation of the 5-fluorouracil plasma level in patients with colorectal cancer undergoing continuous infusion chemotherapy
Abstract
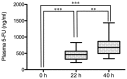
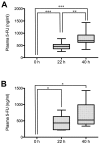
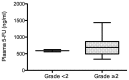
5-Fluorouracil (5-FU) dosing has traditionally been based on the body surface area (BSA) in colorectal cancer treatment. However, there is accumulating evidence that dosing based on BSA may be of limited use. The purpose of the present study was to evaluate the changes in 5-FU plasma levels and tumor response as well as the severity of adverse events in patients with cancer treated with 5-FU combined chemotherapy. The dosing amount of 5-FU was determined based on the BSA. Blood samples were collected, and 5-FU plasma levels in 15 patients with colorectal cancer were measured three times (0, 22 and 40 h before and after the start of infusion) during constant-infusion of 5-FU for 46 h by an immunoassay. 5-FU plasma levels were significantly higher at 22 and 40 h compared with at 0 h (P<0.001), when all 15 patients were analyzed. Notably, the tumor response of the partial response/stable disease group showed significant increases in 5-FU plasma levels at 40 h compared with at 22 h (P<0.01), while the progressive disease group showed no significant increase. In addition, the 5-FU plasma level in the adverse event level of grade ≥2 was higher than that of grade <2 at 40 h after the start of infusion. Collectively, these observations indicated that during continuous infusion of 5-FU, the 5-FU plasma level increased significantly, and the tumor response (such as partial response, stable or progressive disease) may be influenced by the increase of 5-FU plasma level from the start of infusion. Therefore, the 5-FU plasma level may be a predictive factor for maximizing the tumor response and minimizing the risk of severe adverse events.
Keywords: 5-fluorouracil; adverse events; chemotherapy; colorectal cancer; plasma level; tumor response.
Figures
References
-
- de Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, Boni C, Cortes-Funes H, Cervantes A, Freyer G, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18:2938–2947. doi: 10.1200/JCO.2000.18.16.2938. - DOI - PubMed
LinkOut - more resources
Full Text Sources
