Single-Cell RNA Sequencing of Visceral Adipose Tissue Leukocytes Reveals that Caloric Restriction Following Obesity Promotes the Accumulation of a Distinct Macrophage Population with Features of Phagocytic Cells
- PMID: 31396408
- PMCID: PMC6687332
- DOI: 10.20900/immunometab20190008
Single-Cell RNA Sequencing of Visceral Adipose Tissue Leukocytes Reveals that Caloric Restriction Following Obesity Promotes the Accumulation of a Distinct Macrophage Population with Features of Phagocytic Cells
Abstract
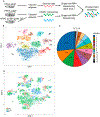
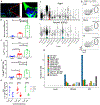
Obesity can lead to type 2 diabetes and is an epidemic. A major contributor to its adverse effects is inflammation of the visceral adipose tissue (VAT). Life-long caloric restriction (CR), in contrast, results in extended lifespan, enhanced glucose tolerance/insulin sensitivity, and other favorable phenotypes. The effects of CR following obesity are incompletely established, but studies show multiple benefits. Many leukocyte types, macrophages predominantly, reside in VAT in homeostatic and pathological states. CR following obesity transiently increases VAT macrophage content prior to resolution of inflammation and obesity, suggesting that macrophage content and phenotype play critical roles. Here, we examined the heterogeneity of VAT leukocytes and the effects of obesity and CR. In general, our single-cell RNA-sequencing data demonstrate that macrophages are the most abundant and diverse subpopulation of leukocytes in VAT. Obesity induced significant transcriptional changes in all 15 leukocyte subpopulations, with many genes showing coordinated changes in expression across the leukocyte subpopulations. Additionally, obese VAT displayed expansion of one major macrophage subpopulation, which, in silico, was enriched in lipid binding and metabolic processes. This subpopulation returned from dominance in obesity to lean proportions after only 2 weeks of CR, although the pattern of gene expression overall remained similar. Surprisingly, CR VAT is dominated by a different macrophage subpopulation, which is absent in lean conditions. This subpopulation is enriched in genes related to phagocytosis and we postulate that its function includes clearance of dead cells, as well as excess lipids, contributing to limiting VAT inflammation and restoration of the homeostatic state.
Keywords: adipose tissue; caloric-restriction; heterogeneity; leukocytes; macrophages; obesity; phagocytosis; single-cell RNA-seq; weight loss.
Conflict of interest statement
CONFLICT OF INTEREST The authors declare that there are no conflicts of interest.
Figures




References
-
- Wu H, Ghosh S, Perrard XD, Feng L, Garcia GE, Perrard JL, et al. T-cell accumulation and regulated on activation, normal T cell expressed and secreted upregulation in adipose tissue in obesity. Circulation. 2007;115(8):1029–38. - PubMed
-
- Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219–46. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
