From cortex to cord: motor circuit plasticity after spinal cord injury
- PMID: 31397332
- PMCID: PMC6788232
- DOI: 10.4103/1673-5374.262572
From cortex to cord: motor circuit plasticity after spinal cord injury
Abstract
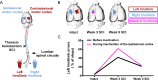
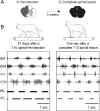
Spinal cord injury is associated with chronic sensorimotor deficits due to the interruption of ascending and descending tracts between the brain and spinal cord. Functional recovery after anatomically complete spinal cord injury is limited due to the lack of long-distance axonal regeneration of severed fibers in the adult central nervous system. Most spinal cord injuries in humans, however, are anatomically incomplete. Although restorative treatment options for spinal cord injury remain currently limited, research from experimental models of spinal cord injury have revealed a tremendous capability for both spontaneous and treatment-induced plasticity of the corticospinal system that supports functional recovery. We review recent advances in the understanding of corticospinal circuit plasticity after spinal cord injury and concentrate mainly on the hindlimb motor cortex, its corticospinal projections, and the role of spinal mechanisms that support locomotor recovery. First, we discuss plasticity that occurs at the level of motor cortex and the reorganization of cortical movement representations. Next, we explore downstream plasticity in corticospinal projections. We then review the role of spinal mechanisms in locomotor recovery. We conclude with a perspective on harnessing neuroplasticity with therapeutic interventions to promote functional recovery.
Keywords: animal models; corticospinal tract; forelimb; functional recovery; hindlimb; locomotion; motor cortex; motor map; neuroplasticity; spinal cord injury.
Conflict of interest statement
None
Figures
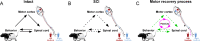
References
-
- Antri M, Barthe JY, Mouffle C, Orsal D. Long-lasting recovery of locomotor function in chronic spinal rat following chronic combined pharmacological stimulation of serotonergic receptors with 8-OHDPAT and quipazine. Neurosci Lett. 2005;384:162–167. - PubMed
-
- Armand J. The origin course and terminations of corticospinal fibers in various mammals. Prog Brain Res. 1982;57:329–360. - PubMed
-
- Asanuma H, Sakata H. Functional organization of a cortical efferent system examined with focal depth stimulation in cats. J Neurophysiol. 1967;30:35–54.
-
- Asboth L, Friedli L, Beauparlant J, Martinez-Gonzalez C, Anil S, Rey E, Baud L, Pidpruzhnykova G, Anderson MA, Shkorbatova P, Batti L, Pages S, Kreider J, Schneider BL, Barraud Q, Courtine G. Cortico-reticulo-spinal circuit reorganization enables functional recovery after severe spinal cord contusion. Nat Neurosci. 2018;21:576–588. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Medical

