A randomized, phase 1b study of the pharmacokinetics, pharmacodynamics, safety, and tolerability of bleselumab, a fully human, anti-CD40 monoclonal antibody, in kidney transplantation
- PMID: 31397943
- PMCID: PMC6972670
- DOI: 10.1111/ajt.15560
A randomized, phase 1b study of the pharmacokinetics, pharmacodynamics, safety, and tolerability of bleselumab, a fully human, anti-CD40 monoclonal antibody, in kidney transplantation
Abstract
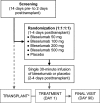
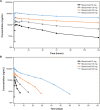
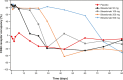
This study evaluated the safety, tolerability, pharmacokinetics, and pharmacodynamics of various doses of the anti-CD40 monoclonal antibody bleselumab (ASKP1240) in de novo kidney transplant recipients receiving concomitant standard immunosuppression over 90 days posttransplant. Transplant recipients were randomized (1:1:1:1:1) to bleselumab 50 mg, 100 mg, 200 mg, or 500 mg, or placebo, in addition to standard maintenance immunosuppression. The primary pharmacokinetic endpoints were AUCinf , Cmax , and AUClast . The primary pharmacodynamic endpoint was B cell CD40 receptor occupancy over time. Overall, 50 kidney transplant recipients were randomized; 45 received their randomized treatment (bleselumab [n = 37] or placebo [n = 8]). AUCinf and AUClast demonstrated a more than dose-proportional increase in the range of 50-500 mg, and Cmax increased linearly with increasing dose. Maximal receptor occupancy for B cell CD40 was reached at all dose levels and was prolonged as dose increased. No kidney transplant recipients experienced cytokine release syndrome or a thromboembolic event. Treatment-emergent anti-bleselumab antibodies were found in one kidney transplant recipient in the bleselumab 50 mg group; these were detected only at Day 7. Overall, bleselumab demonstrated nonlinear pharmacokinetics and dose-dependent prolonged B cell CD40 receptor occupancy and was well tolerated at all doses (ClinicalTrials.gov: NCT01279538).
Keywords: antibody biology; clinical research/practice; kidney transplantation/nephrology; kidney transplantation: living donor.
© 2019 The Authors. American Journal of Transplantation published by Wiley Periodicals, Inc. on behalf of The American Society of Transplantation and the American Society of Transplant Surgeons.
Figures

Similar articles
-
Efficacy and safety of bleselumab in kidney transplant recipients: A phase 2, randomized, open-label, noninferiority study.Am J Transplant. 2020 Jan;20(1):159-171. doi: 10.1111/ajt.15591. Epub 2019 Oct 19. Am J Transplant. 2020. PMID: 31509331 Clinical Trial.
-
Efficacy and Safety of Bleselumab in Preventing the Recurrence of Primary Focal Segmental Glomerulosclerosis in Kidney Transplant Recipients: A Phase 2a, Randomized, Multicenter Study.Transplantation. 2024 Aug 1;108(8):1782-1792. doi: 10.1097/TP.0000000000004985. Epub 2024 Jul 20. Transplantation. 2024. PMID: 39042770 Free PMC article. Clinical Trial.
-
Randomized, controlled study of bleselumab (ASKP1240) pharmacokinetics and safety in patients with moderate-to-severe plaque psoriasis.Biopharm Drug Dispos. 2018 May;39(5):245-255. doi: 10.1002/bdd.2130. Biopharm Drug Dispos. 2018. PMID: 29679478 Free PMC article. Clinical Trial.
-
Developments in immunosuppression.Curr Opin Organ Transplant. 2021 Feb 1;26(1):91-96. doi: 10.1097/MOT.0000000000000844. Curr Opin Organ Transplant. 2021. PMID: 33332922 Review.
-
Optimizing the clinical utility of sirolimus-based immunosuppression for kidney transplantation.Clin Transplant. 2019 Feb;33(2):e13464. doi: 10.1111/ctr.13464. Epub 2019 Jan 2. Clin Transplant. 2019. PMID: 30548896 Review.
Cited by
-
CD40/CD40L Signaling as a Promising Therapeutic Target for the Treatment of Renal Disease.J Clin Med. 2020 Nov 13;9(11):3653. doi: 10.3390/jcm9113653. J Clin Med. 2020. PMID: 33202988 Free PMC article. Review.
-
Costimulation Blockade in Kidney Transplant Recipients.Drugs. 2020 Jan;80(1):33-46. doi: 10.1007/s40265-019-01226-6. Drugs. 2020. PMID: 31749062 Free PMC article. Review.
-
Genetically engineered IgG1 and nanobody oligomers acquire strong intrinsic CD40 agonism.Bioengineered. 2024 Dec;15(1):2302246. doi: 10.1080/21655979.2024.2302246. Epub 2024 Jan 12. Bioengineered. 2024. PMID: 38214443 Free PMC article.
-
Exploring Costimulatory Blockade-Based Immunologic Strategies in Transplantation: Are They a Promising Immunomodulatory Approach for Organ and Vascularized Composite Allotransplantation?J Pers Med. 2024 Mar 20;14(3):322. doi: 10.3390/jpm14030322. J Pers Med. 2024. PMID: 38541064 Free PMC article. Review.
-
Integrative bioinformatics frameworks for abdominal aortic aneurysm using GWAS meta-analysis, biological network construction, and structural modeling.Sci Rep. 2025 Jul 1;15(1):22331. doi: 10.1038/s41598-025-07989-1. Sci Rep. 2025. PMID: 40595262 Free PMC article.
References
-
- Riley JL, June CH. The CD28 family: a T‐cell rheostat for therapeutic control of T‐cell activation. Blood. 2005;105(1):13‐21. - PubMed
-
- Vincenti F, Charpentier B, Vanrenterghem Y, et al. A phase III study of belatacept‐based immunosuppression regimens versus cyclosporine in renal transplant recipients (BENEFIT study). Am J Transplant. 2010;10(3):535‐546. - PubMed
-
- Durrbach A, Pestana JM, Pearson T, et al. A phase III study of belatacept versus cyclosporine in kidney transplants from extended criteria donors (BENEFIT‐EXT study). Am J Transplant. 2010;10(3):547‐557. - PubMed
-
- FDA . Belatacept prescribing information. https://packageinserts.bms.com/pi/pi_nulojix.pdf. Accessed December 2018.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

