The Oncogenic Action of NRF2 Depends on De-glycation by Fructosamine-3-Kinase
- PMID: 31398338
- PMCID: PMC6693658
- DOI: 10.1016/j.cell.2019.07.031
The Oncogenic Action of NRF2 Depends on De-glycation by Fructosamine-3-Kinase
Abstract
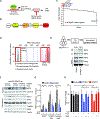
The NRF2 transcription factor controls a cell stress program that is implicated in cancer and there is great interest in targeting NRF2 for therapy. We show that NRF2 activity depends on Fructosamine-3-kinase (FN3K)-a kinase that triggers protein de-glycation. In its absence, NRF2 is extensively glycated, unstable, and defective at binding to small MAF proteins and transcriptional activation. Moreover, the development of hepatocellular carcinoma triggered by MYC and Keap1 inactivation depends on FN3K in vivo. N-acetyl cysteine treatment partially rescues the effects of FN3K loss on NRF2 driven tumor phenotypes indicating a key role for NRF2-mediated redox balance. Mass spectrometry reveals that other proteins undergo FN3K-sensitive glycation, including translation factors, heat shock proteins, and histones. How glycation affects their functions remains to be defined. In summary, our study reveals a surprising role for the glycation of cellular proteins and implicates FN3K as targetable modulator of NRF2 activity in cancer.
Keywords: EGFR; FN3K; KEAP1; NRF2; de-glycation; fructosamine; glucose; glycation; hepatocellular carcinoma; redox.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests
The authors declare no competing interests.
Figures
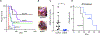





References
-
- Abdel-Wahab YH, O’Harte FP, Barnett CR, and Flatt PR (1997). Characterization of insulin glycation in insulin-secreting cells maintained in tissue culture. The Journal of endocrinology 152, 59–67. - PubMed
-
- Arbour KC, Jordan E, Kim HR, Dienstag J, Yu HA, Sanchez-Vega F, Lito P, Berger M, Solit DB, Hellmann M, et al. (2018). Effects of Co-occurring Genomic Alterations on Outcomes in Patients with KRAS-Mutant Non-Small Cell Lung Cancer. Clinical cancer research : an official journal of the American Association for Cancer Research 24, 334–340. - PMC - PubMed
-
- Bai X, Chen Y, Hou X, Huang M, and Jin J (2016). Emerging role of NRF2 in chemoresistance by regulating drug-metabolizing enzymes and efflux transporters. Drug metabolism reviews 48, 541–567. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

