Targeting Peripheral Somatosensory Neurons to Improve Tactile-Related Phenotypes in ASD Models
- PMID: 31398341
- PMCID: PMC6704376
- DOI: 10.1016/j.cell.2019.07.024
Targeting Peripheral Somatosensory Neurons to Improve Tactile-Related Phenotypes in ASD Models
Abstract
Somatosensory over-reactivity is common among patients with autism spectrum disorders (ASDs) and is hypothesized to contribute to core ASD behaviors. However, effective treatments for sensory over-reactivity and ASDs are lacking. We found distinct somatosensory neuron pathophysiological mechanisms underlie tactile abnormalities in different ASD mouse models and contribute to some ASD-related behaviors. Developmental loss of ASD-associated genes Shank3 or Mecp2 in peripheral mechanosensory neurons leads to region-specific brain abnormalities, revealing links between developmental somatosensory over-reactivity and the genesis of aberrant behaviors. Moreover, acute treatment with a peripherally restricted GABAA receptor agonist that acts directly on mechanosensory neurons reduced tactile over-reactivity in six distinct ASD models. Chronic treatment of Mecp2 and Shank3 mutant mice improved body condition, some brain abnormalities, anxiety-like behaviors, and some social impairments but not memory impairments, motor deficits, or overgrooming. Our findings reveal a potential therapeutic strategy targeting peripheral mechanosensory neurons to treat tactile over-reactivity and select ASD-related behaviors.
Keywords: ASD therapeutics; GABA; autism spectrum disorders; brain development; mechanosensation; mouse genetics.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests
LLO and DDG have patents pending on the use of peripherally restricted GABAA receptor drugs for the treatment of tactile over-reactivity in ASD and other disorders.
Figures
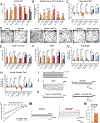
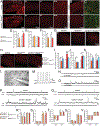

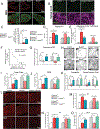


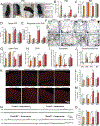
Comment in
-
A Light Touch on Sociability.Cell. 2019 Aug 8;178(4):769-771. doi: 10.1016/j.cell.2019.07.022. Cell. 2019. PMID: 31398332
References
-
- Baio J, Wiggins L, Christensen DL, Maenner MJ, Daniels J, Warren Z, Kurzius-Spencer M, Zahorodny W, Robinson Rosenberg C, White T, et al. (2018). Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years - Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2014. MMWR Surveill Summ 67, 1–23. - PMC - PubMed
-
- Bakker CE, Verheij C, Willemsen R, van der Helm R, Oerlemans F, Vermey M, Bygrave A, Hoogeveen AT, and Oostra BA (1994). Fmr1 knockout mice: a model to study fragile X mental retardation. The Dutch-Belgian Fragile X Consortium. Cell 78, 23–33. - PubMed
-
- Bhattacherjee A, Mu Y, Winter MK, Knapp JR, Eggimann LS, Gunewardena SS, Kobayashi K, Kato S, Krizsan-Agbas D, and Smith PG (2017). Neuronal cytoskeletal gene dysregulation and mechanical hypersensitivity in a rat model of Rett syndrome. Proceedings of the National Academy of Sciences of the United States of America 114, E6952–E6961. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

