CAG Repeat Not Polyglutamine Length Determines Timing of Huntington's Disease Onset
- PMID: 31398342
- PMCID: PMC6700281
- DOI: 10.1016/j.cell.2019.06.036
CAG Repeat Not Polyglutamine Length Determines Timing of Huntington's Disease Onset
Abstract
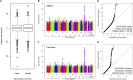
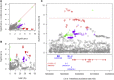
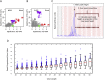
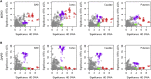
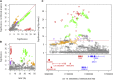
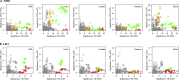
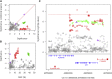
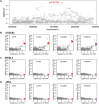
Variable, glutamine-encoding, CAA interruptions indicate that a property of the uninterrupted HTT CAG repeat sequence, distinct from the length of huntingtin's polyglutamine segment, dictates the rate at which Huntington's disease (HD) develops. The timing of onset shows no significant association with HTT cis-eQTLs but is influenced, sometimes in a sex-specific manner, by polymorphic variation at multiple DNA maintenance genes, suggesting that the special onset-determining property of the uninterrupted CAG repeat is a propensity for length instability that leads to its somatic expansion. Additional naturally occurring genetic modifier loci, defined by GWAS, may influence HD pathogenesis through other mechanisms. These findings have profound implications for the pathogenesis of HD and other repeat diseases and question the fundamental premise that polyglutamine length determines the rate of pathogenesis in the "polyglutamine disorders."
Keywords: CAG repeat; DNA maintenance; DNA repair; Huntington’s disease; age at onset; disease modification; genetic modifier; polyglutamine disease; somatic DNA expansion; trinucleotide repeat.
Copyright © 2019 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
J.F.G. and V.C.W. are Scientific Advisory Board members and have financial interests in Triplet Therapeutics, Inc., a company developing new therapeutic approaches to address triplet repeat disorders such Huntington’s Disease and Myotonic Dystrophy. Their interests were reviewed and are managed by Massachusetts General Hospital and Partners HealthCare in accordance with their conflict of interest policies. J.D.L. is an advisory board member for F. Hoffman-La Roche, Wave Life Sciences USA, Huntington Study Group (for uniQuire biopharma B.V.), and Mitoconix Bio and a consultant for Vaccinex and Azevan Pharmaceuticals. V.C.W. is a Scientific Advisory Board member of LoQus23 Therapeutics and receives research support from Pfizer. L.J. is a consultant for LoQus23 Therapeutics. D.G.M. is a Scientific Advisory Board member and has a financial interest in Triplet Therapeutics, Inc. G.B.L. has provided consulting services, advisory board functions, clinical trial services, and/or lectures for Allergan, Alnylam, Amarin, AOP Orphan Pharmaceuticals AG, Bayer Pharma AG, CHDI Foundation, GlaxoSmithKline, Hoffmann-LaRoche, Ipsen, ISIS Pharma, Lundbeck, Neurosearch Inc, Medesis, Medivation, Medtronic, Novartis, Pfizer, Prana Biotechnology, Sangamo/Shire, Siena Biotech, Temmler Pharma GmbH, and Teva Pharmaceuticals.
Figures


References
-
- Aulchenko Y.S., Ripke S., Isaacs A., van Duijn C.M. GenABEL: an R library for genome-wide association analysis. Bioinformatics. 2007;23:1294–1296. - PubMed
-
- Bečanović K., Nørremølle A., Neal S.J., Kay C., Collins J.A., Arenillas D., Lilja T., Gaudenzi G., Manoharan S., Doty C.N., REGISTRY Investigators of the European Huntington’s Disease Network A SNP in the HTT promoter alters NF-κB binding and is a bidirectional genetic modifier of Huntington disease. Nat. Neurosci. 2015;18:807–816. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

