Assessment of long-term neurodevelopmental outcome following trials of medicinal products in newborn infants
- PMID: 31398720
- PMCID: PMC6848023
- DOI: 10.1038/s41390-019-0526-1
Assessment of long-term neurodevelopmental outcome following trials of medicinal products in newborn infants
Abstract
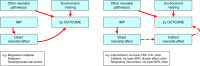
There is significant uncertainty over the role of assessment of long-term neurodevelopmental outcome (LTO) in neonatal clinical trials. A multidisciplinary working group was established to identify key issues in this area and to make recommendations about optimal approaches to evaluate LTO in therapeutic trials in newborns, which can be developed by sponsors and investigators with other key stakeholders. A key consideration for neonatal trials is the potential for the investigational product to cause widespread effects and drives the need to assess outcome in multiple organs. Thus investigators must assess whether the product has an impact on the brain and the potential for it to cause potential effects on LTO. Critically, is assessment of LTO an important direct therapeutic target or a safety outcome? Such decisions and outcomes need to be specific to the product being studied and use published data, only considering expert opinion when prior evidence does not exist. In designing the trial, the balance of benefits, costs, and burdens of assessments to the researcher and families need to be considered. Families and parent advocates should be involved in design and execution of the study. A framework is presented for use by all key stakeholders to determine the need, nature, and duration of LTO assessments in regulatory trials involving newborn infants.
Conflict of interest statement
The authors declare no competing interests.
Figures