Exosomes and Their Noncoding RNA Cargo Are Emerging as New Modulators for Diabetes Mellitus
- PMID: 31398847
- PMCID: PMC6721737
- DOI: 10.3390/cells8080853
Exosomes and Their Noncoding RNA Cargo Are Emerging as New Modulators for Diabetes Mellitus
Abstract
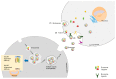
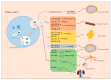
Diabetes belongs to a group of metabolic disorders characterized by long term high blood glucose levels due to either inadequate production of insulin (Type 1 diabetes, T1DM) or poor response of the recipient cell to insulin (Type 2 diabetes, T2DM). Organ dysfunctions are the main causes of morbidity and mortality due to high glucose levels. Understanding the mechanisms of organ crosstalk may help us improve our basic knowledge and find novel strategies to better treat the disease. Exosomes are part of a newly emerged research area and have attracted a great deal of attention for their capacity to regulate communications between cells. In conditions of diabetes, exosomes play important roles in the pathological processes in both T1DM and T2DM, such as connecting the immune cell response to pancreatic tissue injury, as well as adipocyte stimulation to insulin resistance of skeletal muscle or liver. Furthermore, in recent years, nucleic acids containing exosomes-especially microRNAs (miRNAs) and long noncoding RNAs (lncRNAs)-have been shown to mainly regulate communications between organs in pathological processes of diabetes, including influencing metabolic signals and insulin signals in target tissues, affecting cell viability, and modulating inflammatory pancreatic cells. Moreover, exosome miRNAs show promise in their use as biomarkers or in treatments for diabetes and diabetic complications. Thus, this paper summarizes the recent work on exosomes related to diabetes as well as the roles of exosomal miRNAs and lncRNAs in diabetic pathology and diagnosis in order to help us better understand the exact roles of exosomes in diabetes development.
Keywords: exosomes; long noncoding RNA; miRNA; type 1 diabetes; type 2 diabetes.
Conflict of interest statement
The authors confirm that there are no conflicts of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

