Liver Progenitors and Adult Cell Plasticity in Hepatic Injury and Repair: Knowns and Unknowns
- PMID: 31399003
- PMCID: PMC7212705
- DOI: 10.1146/annurev-pathmechdis-012419-032824
Liver Progenitors and Adult Cell Plasticity in Hepatic Injury and Repair: Knowns and Unknowns
Abstract
The liver is a complex organ performing numerous vital physiological functions. For that reason, it possesses immense regenerative potential. The capacity for repair is largely attributable to the ability of its differentiated epithelial cells, hepatocytes and biliary epithelial cells, to proliferate after injury. However, in cases of extreme acute injury or prolonged chronic insult, the liver may fail to regenerate or do so suboptimally. This often results in life-threatening end-stage liver disease for which liver transplantation is the only effective treatment. In many forms of liver injury, bipotent liver progenitor cells are theorized to be activated as an additional tier of liver repair. However, the existence, origin, fate, activation, and contribution to regeneration of liver progenitor cells is hotly debated, especially since hepatocytes and biliary epithelial cells themselves may serve as facultative stem cells for one another during severe liver injury. Here, we discuss the evidence both supporting and refuting the existence of liver progenitor cells in a variety of experimental models. We also debate the validity of developing therapies harnessing the capabilities of these cells as potential treatments for patients with severe and chronic liver diseases.
Keywords: ductular reaction; liver cancer; liver regeneration; liver stem cell; oval cell.
Figures
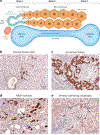
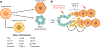

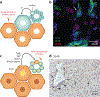
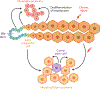
References
-
- Stoick-Cooper CL, Moon RT, Weidinger G. 2007. Advances in signaling in vertebrate regeneration as a prelude to regenerative medicine. Genes Dev. 21:1292–315 - PubMed
-
- Ferretti P, Zhang F, O’Neill P. 2003. Changes in spinal cord regenerative ability through phylogenesis and development: lessons to be learnt. Dev. Dyn 226:245–56 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

