Development of a new fusion-enhanced oncolytic immunotherapy platform based on herpes simplex virus type 1
- PMID: 31399043
- PMCID: PMC6689178
- DOI: 10.1186/s40425-019-0682-1
Development of a new fusion-enhanced oncolytic immunotherapy platform based on herpes simplex virus type 1
Abstract
Background: Oncolytic viruses preferentially replicate in tumors as compared to normal tissue and promote immunogenic cell death and induction of host systemic anti-tumor immunity. HSV-1 was chosen for further development as an oncolytic immunotherapy in this study as it is highly lytic, infects human tumor cells broadly, kills mainly by necrosis and is a potent activator of both innate and adaptive immunity. HSV-1 also has a large capacity for the insertion of additional, potentially therapeutic, exogenous genes. Finally, HSV-1 has a proven safety and efficacy profile in patients with cancer, talimogene laherparepvec (T-VEC), an oncolytic HSV-1 which expresses GM-CSF, being the only oncolytic immunotherapy approach that has received FDA approval. As the clinical efficacy of oncolytic immunotherapy has been shown to be further enhanced by combination with immune checkpoint inhibitors, developing improved oncolytic platforms which can synergize with other existing immunotherapies is a high priority. In this study we sought to further optimize HSV-1 based oncolytic immunotherapy through multiple approaches to maximize: (i) the extent of tumor cell killing, augmenting the release of tumor antigens and danger-associated molecular pattern (DAMP) factors; (ii) the immunogenicity of tumor cell death; and (iii) the resulting systemic anti-tumor immune response.
Methods: To sample the wide diversity amongst clinical strains of HSV-1, twenty nine new clinical strains isolated from cold sores from otherwise healthy volunteers were screened across a panel of human tumor cell lines to identify the strain with the most potent tumor cell killing ability, which was then used for further development. Following deletion of the genes encoding ICP34.5 and ICP47 to provide tumor selectivity, the extent of cell killing and the immunogenicity of cell death was enhanced through insertion of a gene encoding a truncated, constitutively highly fusogenic form of the envelope glycoprotein of gibbon ape leukemia virus (GALV-GP-R-). A number of further armed derivatives of this virus were then constructed intended to further enhance the anti-tumor immune response which was generated following fusion-enhanced, oncolytic virus replication-mediated cell death. These viruses expressed GMCSF, an anti-CTLA-4 antibody-like molecule, CD40L, OX40L and/or 4-1BB, each of which is expected to act predominantly at the site and time of immune response initiation. Expression of these proteins was confirmed by ELISA and/or western blotting. Immunogenic cell death was assessed by measuring the levels of HMGB1 and ATP from cell free supernatants from treated cells, and by measuring the surface expression of calreticulin. GALV-GP-R- mediated cell to cell fusion and killing was tested in a range of tumor cell lines in vitro. Finally, the in vivo therapeutic potential of these viruses was tested using human A549 (lung cancer) and MDA-MB-231(breast cancer) tumor nude mouse xenograft models and systemic anti-tumor effects tested using dual flank syngeneic 4434 (melanoma), A20 (lymphoma) mouse tumor models alone and in combination with a murine anti-PD1 antibody, and 9 L (gliosarcoma) tumors in rats.
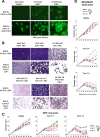
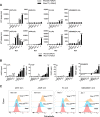
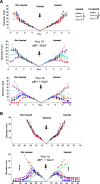
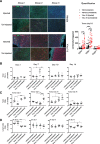
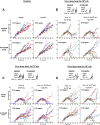
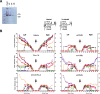
Results: The twenty nine clinical strains of HSV-1 isolated and tested demonstrated a broad range of tumor cell killing abilities allowing the most potent strain to be identified which was then used for further development. Oncolytic ability was demonstrated to be further augmented by the expression of GALV-GP-R- in a range of tumor cell lines in vitro and in mouse xenograft models in nude mice. The expression of GALV-GP-R- was also demonstrated to lead to enhanced immunogenic cell death in vitro as confirmed by the increased release of HMGB1 and ATP and increased levels of calreticulin on the cell surface. Experiments using the rat 9 L syngeneic tumor model demonstrated that GALV-GP-R- expression increased abscopal uninjected (anenestic) tumor responses and data using mouse 4434 tumors demonstrated that virus treatment increased CD8+ T cell levels both in the injected and uninjected tumor, and also led to increased expression of PD-L1. A combination study using varying doses of a virus expressing GALV-GP-R- and mGM-CSF and an anti-murine PD1 antibody showed enhanced anti-tumor effects with the combination which was most evident at low virus doses, and also lead to immunological memory. Finally, treatment of mice with derivatives of this virus which additionally expressed anti-mCTLA-4, mCD40L, m4-1BBL, or mOX40L demonstrated enhanced activity, particularly in uninjected tumors.
Conclusion: The new HSV-1 based platform described provides a potent and versatile approach to developing new oncolytic immunotherapies for clinical use. Each of the modifications employed was demonstrated to aid in optimizing the potential of the virus to both directly kill tumors and to lead to systemic therapeutic benefit. For clinical use, these viruses are expected to be most effective in combination with other anti-cancer agents, in particular PD1/L1-targeted immune checkpoint blockade. The first virus from this program (expressing GALV-GP-R- and hGM-CSF) has entered clinical development alone and in combination with anti-PD1 therapy in a number of tumor types (NCT03767348).
Keywords: Anenestic effect; Herpes simplex-1; Immunotherapy; Oncolytic viruses.
Conflict of interest statement
H.L. Kaufman, R.S. Coffin, P.K. Bommareddy, L. Kunchuria, and S. Thomas are employees of Replimune, Inc.
Figures

References
-
- Hugo W, Zaretsky JM, Sun L, Song C, Moreno BH, Hu-Lieskovan S, Berent-Maoz B, Pang J, Chmielowski B, Cherry G, Seja E, Lomeli S, Kong X, Kelley MC, Sosman JA, Johnson DB, Ribas A, Lo RS. Genomic and transcriptomic features of response to anti-PD-1 therapy in metastatic melanoma. Cell. 2016;165:35–44. doi: 10.1016/j.cell.2016.02.065. - DOI - PMC - PubMed
-
- Andtbacka RH, Kaufman HL, Collichio F, Amatruda T, Senzer N, Chesney J, Delman KA, Spitler LE, Puzanov I, Agarwala SS, Milhem M, Cranmer L, Curti B, Lewis K, Ross M, Guthrie T, Linette GP, Daniels GA, Harrington K, Middleton MR, Miller WH, Jr, Zager JS, Ye Y, Yao B, Li A, Doleman S, VanderWalde A, Gansert J, Coffin RS. Talimogene Laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol. 2015;33:2780–2788. doi: 10.1200/JCO.2014.58.3377. - DOI - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
