Quantitative proteomic analysis reveals AK2 as potential biomarker for late normal tissue radiotoxicity
- PMID: 31399108
- PMCID: PMC6688300
- DOI: 10.1186/s13014-019-1351-8
Quantitative proteomic analysis reveals AK2 as potential biomarker for late normal tissue radiotoxicity
Abstract
Background: Biomarkers for predicting late normal tissue toxicity to radiotherapy are necessary to personalize treatments and to optimize clinical benefit. Many radiogenomic studies have been published on this topic. Conversely, proteomics approaches are not much developed, despite their advantages.
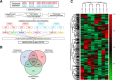
Methods: We used the isobaric tags for relative and absolute quantitation (iTRAQ) proteomic approach to analyze differences in protein expression levels in ex-vivo irradiated (8 Gy) T lymphocytes from patients with grade ≥ 2 radiation-induced breast fibrosis (grade ≥ 2 bf+) and patients with grade < 2 bf + after curative intent radiotherapy. Patients were selected from two prospective clinical trials (COHORT and PHRC 2005) and were used as discovery and confirmation cohorts.
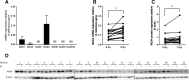
Results: Among the 1979 quantified proteins, 23 fulfilled our stringent biological criteria. Immunoblotting analysis of four of these candidate proteins (adenylate kinase 2, AK2; annexin A1; heat shock cognate 71 kDa protein; and isocitrate dehydrogenase 2) confirmed AK2 overexpression in 8 Gy-irradiated T lymphocytes from patients with grade ≥ 2 bf + compared with patients with grade < 2 bf+. As these candidate proteins are involved in oxidative stress regulation, we also evaluated radiation-induced reactive oxygen species (ROS) production in peripheral blood mononuclear cells from patients with grade ≥ 2 bf + and grade < 2 bf+. Total ROS level, and especially superoxide anion level, increased upon ex-vivo 8 Gy-irradiation in all patients. Analysis of NADPH oxidases (NOXs), a major source of superoxide ion in the cell, showed a significant increase of NOX4 mRNA and protein levels after irradiation in both patient groups. Conversely, only NOX4 mRNA level was significantly different between groups (grade ≥ 2 bf + and grade < 2 bf+).
Conclusion: These findings identify AK2 as a potential radiosensitivity candidate biomarker. Overall, our proteomic approach highlights the important role of oxidative stress in late radiation-induced toxicity, and paves the way for additional studies on NOXs and superoxide ion metabolism.
Keywords: AK2; NADPH oxidases; Normal tissue radiotoxicity; Proteomics; Radiation-induced breast fibrosis; Radiosensitivity; Radiotherapy.
Conflict of interest statement
ICM, CHU Montpellier and University of Montpellier own a patent (WO2014154854A1) associated with a method for determining radiosensitivity that includes the protein AK2. D. A., J. L., J. S., and A.M. are the inventors. The other authors have no conflict of interest to declare.
Figures


Similar articles
-
Radiation-induced CD8 T-lymphocyte Apoptosis as a Predictor of Breast Fibrosis After Radiotherapy: Results of the Prospective Multicenter French Trial.EBioMedicine. 2015 Oct 25;2(12):1965-73. doi: 10.1016/j.ebiom.2015.10.024. eCollection 2015 Dec. EBioMedicine. 2015. PMID: 26844275 Free PMC article. Clinical Trial.
-
Partial breast irradiation with interstitial 60CO brachytherapy results in frequent grade 3 or 4 toxicity. Evidence based on a 12-year follow-up of 70 patients.Int J Radiat Oncol Biol Phys. 2004 Mar 15;58(4):1022-33. doi: 10.1016/j.ijrobp.2003.08.013. Int J Radiat Oncol Biol Phys. 2004. PMID: 15001241
-
NRG Oncology-Radiation Therapy Oncology Group Study 1014: 1-Year Toxicity Report From a Phase 2 Study of Repeat Breast-Preserving Surgery and 3-Dimensional Conformal Partial-Breast Reirradiation for In-Breast Recurrence.Int J Radiat Oncol Biol Phys. 2017 Aug 1;98(5):1028-1035. doi: 10.1016/j.ijrobp.2017.03.016. Epub 2017 Mar 18. Int J Radiat Oncol Biol Phys. 2017. PMID: 28721885 Free PMC article. Clinical Trial.
-
A Review of Radiation-Induced Lymphocyte Apoptosis as a Predictor of Late Toxicity After Breast Radiotherapy.J Med Imaging Radiat Sci. 2019 Jun;50(2):337-344. doi: 10.1016/j.jmir.2019.02.004. Epub 2019 Apr 15. J Med Imaging Radiat Sci. 2019. PMID: 31176443
-
[Individual modification of the dose, volume and fractionation of breast radiotherapy].Cancer Radiother. 2019 Oct;23(6-7):778-783. doi: 10.1016/j.canrad.2019.06.004. Epub 2019 Aug 1. Cancer Radiother. 2019. PMID: 31378461 Review. French.
Cited by
-
-Omics potential of in vitro skin models for radiation exposure.Cell Mol Life Sci. 2022 Jul 1;79(7):390. doi: 10.1007/s00018-022-04394-z. Cell Mol Life Sci. 2022. PMID: 35776214 Free PMC article. Review.
-
Multi-Omic Profiling of Multi-Biosamples Reveals the Role of Amino Acid and Nucleotide Metabolism in Endometrial Cancer.Front Oncol. 2022 Apr 29;12:861142. doi: 10.3389/fonc.2022.861142. eCollection 2022. Front Oncol. 2022. PMID: 35574395 Free PMC article.
-
Human coilin interacting nuclear ATPase protein in cancer: uncovering new insights into pathogenesis and therapy.Am J Transl Res. 2020 Jul 15;12(7):4051-4058. eCollection 2020. Am J Transl Res. 2020. PMID: 32774758 Free PMC article.
-
Ionizing Radiation Protein Biomarkers in Normal Tissue and Their Correlation to Radiosensitivity: A Systematic Review.J Pers Med. 2021 Feb 19;11(2):140. doi: 10.3390/jpm11020140. J Pers Med. 2021. PMID: 33669522 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

