Progression criteria in trials with an internal pilot: an audit of publicly funded randomised controlled trials
- PMID: 31399148
- PMCID: PMC6688224
- DOI: 10.1186/s13063-019-3578-y
Progression criteria in trials with an internal pilot: an audit of publicly funded randomised controlled trials
Abstract
Background: With millions of pounds spent annually on medical research in the UK, it is important that studies are spending funds wisely. Internal pilots offer the chance to stop a trial early if it becomes apparent that the study will not be able to recruit enough patients to show whether an intervention is clinically effective. This study aims to assess the use of internal pilots in individually randomised controlled trials funded by the Health Technology Assessment (HTA) programme and to summarise the progression criteria chosen in these trials.
Methods: Studies were identified from reports of the HTA committees' funding decisions from 2012 to 2016. In total, 242 trials were identified of which 134 were eligible to be included in the audit. Protocols for the eligible studies were located on the NIHR Journals website, and if protocols were not available online then study managers were contacted to provide information.
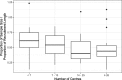
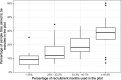
Results: Over two-thirds (72.4%) of studies said in their protocol that they would include an internal pilot phase for their study and 37.8% of studies without an internal pilot had done an external pilot study to assess the feasibility of the full study. A typical study with an internal pilot has a target sample size of 510 over 24 months and aims to recruit one-fifth of their total target sample size within the first one-third of their recruitment time. There has been an increase in studies adopting a three-tiered structure for their progression rules in recent years, with 61.5% (16/26) of studies using the system in 2016 compared to just 11.8% (2/17) in 2015. There was also a rise in the number of studies giving a target recruitment rate in their progression criteria: 42.3% (11/26) in 2016 compared to 35.3% (6/17) in 2015.
Conclusions: Progression criteria for an internal pilot are usually well specified but targets vary widely. For the actual criteria, red/amber/green systems have increased in popularity in recent years. Trials should justify the targets they have set, especially where targets are low.
Keywords: Audit; Feasibility; Internal pilot; Recruitment.
Conflict of interest statement
SG is the Deputy Director of the NIHR HTA programme and Chair of the NIHR HTA commissioning committee. EH and SJ declare that they have no competing interests.
Figures




References
-
- NIHR. National Institute for Health Research Annual Report 2015/16; 2016. Available from: https://www.nihr.ac.uk/documents/about-us/our-contribution-to-research/r.... Accessed 30 July 2019.
-
- NIHR. NIHR Research for Patient Benefit (RfPB) Programme Guidance on Applying for Feasibility Studies; 2017. Available from: https://www.nihr.ac.uk/documents/nihr-research-for-patient-benefit-rfpb-.... Accessed 31 July 2019.
-
- NIHR HTA. Supporting Information for Applicants Applying to the HTA Programme; 2017. Available from: https://www.nihr.ac.uk/documents/hta-supporting-information/11929#Feasib.... Accessed 31 July 2019.
-
- Walters SJ, Bonacho dos Anjos Henriques-Cadby I, Bortolami O, Flight L, Hind D, Jacques RM, et al. Recruitment and retention of participants in randomised controlled trials: a review of trials funded and published by the United Kingdom Health Technology Assessment Programme. BMJ Open. 2017;7(3):e015276. doi: 10.1136/bmjopen-2016-015276. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

