Harmonizing Experimental Data with Modeling to Predict Membrane Protein Insertion in Yeast
- PMID: 31399214
- PMCID: PMC6712491
- DOI: 10.1016/j.bpj.2019.07.013
Harmonizing Experimental Data with Modeling to Predict Membrane Protein Insertion in Yeast
Abstract
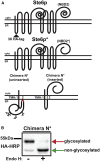
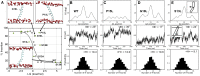
Membrane proteins must adopt their proper topologies within biological membranes, but achieving the correct topology is compromised by the presence of marginally hydrophobic transmembrane helices (TMHs). In this study, we report on a new model membrane protein in yeast that harbors two TMHs fused to an unstable nucleotide-binding domain. Because the second helix (TMH2) in this reporter has an unfavorable predicted free energy of insertion, we employed established methods to generate variants that alter TMH2 insertion free energy. We first found that altering TMH2 did not significantly affect the extent of protein degradation by the cellular quality control machinery. Next, we correlated predicted insertion free energies from a knowledge-based energy scale with the measured apparent free energies of TMH2 insertion. Although the predicted and apparent insertion energies showed a similar trend, the predicted free-energy changes spanned an unanticipated narrow range. By instead using a physics-based model, we obtained a broader range of free energies that agreed considerably better with the magnitude of the experimentally derived values. Nevertheless, some variants still inserted better in yeast than predicted from energy-based scales. Therefore, molecular dynamics simulations were performed and indicated that the corresponding mutations induced conformational changes within TMH2, which altered the number of stabilizing hydrogen bonds. Together, our results offer insight into the ability of the cellular quality control machinery to recognize conformationally distinct misfolded topomers, provide a model to assess TMH insertion in vivo, and indicate that TMH insertion energy scales may be limited depending on the specific protein and the mutation present.
Copyright © 2019 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures



References
-
- Hampton R.Y., Sommer T. Finding the will and the way of ERAD substrate retrotranslocation. Curr. Opin. Cell Biol. 2012;24:460–466. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

