Lactate dehydrogenase and glycerol-3-phosphate dehydrogenase cooperatively regulate growth and carbohydrate metabolism during Drosophila melanogaster larval development
- PMID: 31399469
- PMCID: PMC6765128
- DOI: 10.1242/dev.175315
Lactate dehydrogenase and glycerol-3-phosphate dehydrogenase cooperatively regulate growth and carbohydrate metabolism during Drosophila melanogaster larval development
Abstract
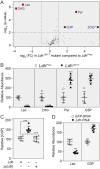
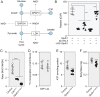
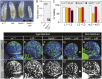
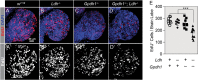
The dramatic growth that occurs during Drosophila larval development requires rapid conversion of nutrients into biomass. Many larval tissues respond to these biosynthetic demands by increasing carbohydrate metabolism and lactate dehydrogenase (LDH) activity. The resulting metabolic program is ideally suited for synthesis of macromolecules and mimics the manner by which cancer cells rely on aerobic glycolysis. To explore the potential role of Drosophila LDH in promoting biosynthesis, we examined how Ldh mutations influence larval development. Our studies unexpectedly found that Ldh mutants grow at a normal rate, indicating that LDH is dispensable for larval biomass production. However, subsequent metabolomic analyses suggested that Ldh mutants compensate for the inability to produce lactate by generating excess glycerol-3-phosphate (G3P), the production of which also influences larval redox balance. Consistent with this possibility, larvae lacking both LDH and G3P dehydrogenase (GPDH1) exhibit growth defects, synthetic lethality and decreased glycolytic flux. Considering that human cells also generate G3P upon inhibition of lactate dehydrogenase A (LDHA), our findings hint at a conserved mechanism in which the coordinate regulation of lactate and G3P synthesis imparts metabolic robustness to growing animal tissues.
Keywords: Aerobic glycolysis; Drosophila; Glycerol-3-phosphate dehydrogenase; Lactate dehydrogenase; Redox balance.
© 2019. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures



References
-
- Backhaus B., Sulkowski E. and Schlote F. (1984). A semi-synthetic, general-purpose medium for Drosophila melanogaster. Dros. Inf. Serv. 60, 210-212.
-
- Billiard J., Dennison J. B., Briand J., Annan R. S., Chai D., Colón M., Dodson C. S., Gilbert S. A., Greshock J., Jing J. et al. (2013). Quinoline 3-sulfonamides inhibit lactate dehydrogenase A and reverse aerobic glycolysis in cancer cells. Cancer Metab. 1, 19 10.1186/2049-3002-1-19 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

