The Intensity of Manganese Deficiency Strongly Affects Root Endodermal Suberization and Ion Homeostasis
- PMID: 31399491
- PMCID: PMC6776859
- DOI: 10.1104/pp.19.00507
The Intensity of Manganese Deficiency Strongly Affects Root Endodermal Suberization and Ion Homeostasis
Abstract
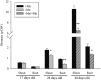
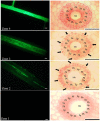
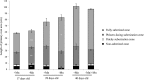
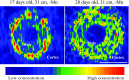
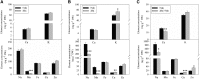
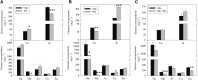
Manganese (Mn) deficiency affects various processes in plant shoots. However, the functions of Mn in roots and the processes involved in root adaptation to Mn deficiency are largely unresolved. Here, we show that the suberization of endodermal cells in barley (Hordeum vulgare) roots is altered in response to Mn deficiency, and that the intensity of Mn deficiency ultimately determines whether suberization increases or decreases. Mild Mn deficiency increased the length of the unsuberized zone close to the root tip, and increased the distance from the root tip at which the fully suberized zone developed. By contrast, strong Mn deficiency increased suberization closer to the root tip. Upon Mn resupply, suberization was identical to that seen on Mn-replete plants. Bioimaging and xylem sap analyses suggest that the reduced suberization in mildly Mn-deficient plants promotes radial Mn transport across the endodermis at a greater distance from the root tip. Less suberin also favors the inwards radial transport of calcium and sodium, but negatively affects the potassium concentration in the stele. During strong Mn deficiency, Mn uptake was directed toward the root tip. Enhanced suberization provides a mechanism to prevent absorbed Mn from leaking out of the stele. With more suberin, the inward radial transport of calcium and sodium decreases, whereas that of potassium increases. We conclude that changes in suberization in response to the intensity of Mn deficiency have a strong effect on root ion homeostasis and ion translocation.
© 2019 American Society of Plant Biologists. All Rights Reserved.
Figures




References
-
- Alassimone J, Fujita S, Doblas VG, van Dop M, Barberon M, Kalmbach L, Vermeer JE, Rojas-Murcia N, Santuari L, Hardtke CS, et al. (2016) Polarly localized kinase SGN1 is required for Casparian strip integrity and positioning. Nat Plants 2: 16113. - PubMed
-
- Andersen TG, Barberon M, Geldner N (2015) Suberization—the second life of an endodermal cell. Curr Opin Plant Biol 28: 9–15 - PubMed
-
- Barberon M, Vermeer JE, De Bellis D, Wang P, Naseer S, Andersen TG, Humbel BM, Nawrath C, Takano J, Salt DE, et al. (2016) Adaptation of root function by nutrient-induced plasticity of endodermal differentiation. Cell 164: 447–459 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

