A Computational Model of Oxytocin Modulation of Olfactory Recognition Memory
- PMID: 31399493
- PMCID: PMC6727149
- DOI: 10.1523/ENEURO.0201-19.2019
A Computational Model of Oxytocin Modulation of Olfactory Recognition Memory
Abstract
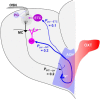
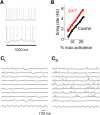
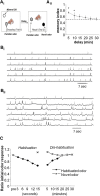
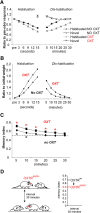
Social recognition in mammals depends on complex interactions between sensory and other brain areas as well as modulatory inputs by specific neuropeptides such as oxytocin (OXT). Social recognition memory specifically has been shown to depend among others on olfactory processing, and can be probed using methods similar to those used when probing non-social odor memory. We here use a computational model of two interconnected olfactory networks in the mouse, the olfactory bulb (OB) and anterior olfactory nucleus, to propose a mechanism for olfactory short-term recognition memory and its modulation in social situations. Based on previous experiments, we propose one early locus for memory to be the OB. During social encounters in mice, pyramidal cells in the anterior olfactory nucleus, themselves driven by olfactory input, are rendered more excitable by OXT release, resulting in stronger feedback to OB local interneurons. This additional input to the OB creates stronger dynamics and improves signal-to-noise ratio of odor responses in the OB proper. As a consequence, mouse social olfactory memories are more strongly encoded and their duration is modulated.
Keywords: anterior olfactory nucleus; computation; olfaction; oxytocin; plasticity; social odors.
Copyright © 2019 Linster and Kelsch.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
