Rgma-Induced Neo1 Proteolysis Promotes Neural Tube Morphogenesis
- PMID: 31399534
- PMCID: PMC6750935
- DOI: 10.1523/JNEUROSCI.3262-18.2019
Rgma-Induced Neo1 Proteolysis Promotes Neural Tube Morphogenesis
Abstract
Neuroepithelial cell (NEC) elongation is one of several key cell behaviors that mediate the tissue-level morphogenetic movements that shape the neural tube (NT), the precursor of the brain and spinal cord. However, the upstream signals that promote NEC elongation have been difficult to tease apart from those regulating apico-basal polarity and hingepoint formation, due to their confounding interdependence. The Repulsive Guidance Molecule a (Rgma)/Neogenin 1 (Neo1) signaling pathway plays a conserved role in NT formation (neurulation) and is reported to regulate both NEC elongation and apico-basal polarity, through signal transduction events that have not been identified. We examine here the role of Rgma/Neo1 signaling in zebrafish (sex unknown), an organism that does not use hingepoints to shape its hindbrain, thereby enabling a direct assessment of the role of this pathway in NEC elongation. We confirm that Rgma/Neo1 signaling is required for microtubule-mediated NEC elongation, and demonstrate via cell transplantation that Neo1 functions cell autonomously to promote elongation. However, in contrast to previous findings, our data do not support a role for this pathway in establishing apical junctional complexes. Last, we provide evidence that Rgma promotes Neo1 glycosylation and intramembrane proteolysis, resulting in the production of a transient, nuclear intracellular fragment (NeoICD). Partial rescue of Neo1a and Rgma knockdown embryos by overexpressing neoICD suggests that this proteolytic cleavage is essential for neurulation. Based on these observations, we propose that RGMA-induced NEO1 proteolysis orchestrates NT morphogenesis by promoting NEC elongation independently of the establishment of apical junctional complexes.SIGNIFICANCE STATEMENT The neural tube, the CNS precursor, is shaped during neurulation. Neural tube defects occur frequently, yet underlying genetic risk factors are poorly understood. Neuroepithelial cell (NEC) elongation is essential for proper completion of neurulation. Thus, connecting NEC elongation with the molecular pathways that control this process is expected to reveal novel neural tube defect risk factors and increase our understanding of NT development. Effectors of cell elongation include microtubules and microtubule-associated proteins; however, upstream regulators remain controversial due to the confounding interdependence of cell elongation and establishment of apico-basal polarity. Here, we reveal that Rgma-Neo1 signaling controls NEC elongation independently of the establishment of apical junctional complexes and identify Rgma-induced Neo1 proteolytic cleavage as a key upstream signaling event.
Keywords: Rgma; cell elongation; microtubules; neogenin; neural tube; regulated intramembrane proteolysis.
Copyright © 2019 the authors.
Figures
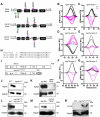
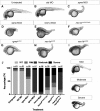








References
-
- Anderson JL, Mulligan TS, Shen MC, Wang H, Scahill CM, Tan FJ, Du SJ, Busch-Nentwich EM, Farber SA (2017) mRNA processing in mutant zebrafish lines generated by chemical and CRISPR-mediated mutagenesis produces unexpected transcripts that escape nonsense-mediated decay. PLOS Genet 13:e1007105. 10.1371/journal.pgen.1007105 - DOI - PMC - PubMed
-
- Avila J, Domínguez J, Díaz-Nido J (1994) Regulation of microtubule dynamics by microtubule-associated protein expression and phosphorylation during neuronal development. Int J Dev Biol 38:13–25. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
