ARF6 and AMAP1 are major targets of KRAS and TP53 mutations to promote invasion, PD-L1 dynamics, and immune evasion of pancreatic cancer
- PMID: 31399545
- PMCID: PMC6717289
- DOI: 10.1073/pnas.1901765116
ARF6 and AMAP1 are major targets of KRAS and TP53 mutations to promote invasion, PD-L1 dynamics, and immune evasion of pancreatic cancer
Abstract
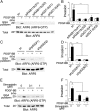
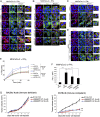
Although KRAS and TP53 mutations are major drivers of pancreatic ductal adenocarcinoma (PDAC), the incurable nature of this cancer still remains largely elusive. ARF6 and its effector AMAP1 are often overexpressed in different cancers and regulate the intracellular dynamics of integrins and E-cadherin, thus promoting tumor invasion and metastasis when ARF6 is activated. Here we show that the ARF6-AMAP1 pathway is a major target by which KRAS and TP53 cooperatively promote malignancy. KRAS was identified to promote eIF4A-dependent ARF6 mRNA translation, which contains a quadruplex structure at its 5'-untranslated region, by inducing TEAD3 and ETV4 to suppress PDCD4; and also eIF4E-dependent AMAP1 mRNA translation, which contains a 5'-terminal oligopyrimidine-like sequence, via up-regulating mTORC1. TP53 facilitated ARF6 activation by platelet-derived growth factor (PDGF), via its known function to promote the expression of PDGF receptor β (PDGFRβ) and enzymes of the mevalonate pathway (MVP). The ARF6-AMAP1 pathway was moreover essential for PDGF-driven recycling of PD-L1, in which KRAS, TP53, eIF4A/4E-dependent translation, mTOR, and MVP were all integral. We moreover demonstrated that the mouse PDAC model KPC cells, bearing KRAS/TP53 mutations, express ARF6 and AMAP1 at high levels and that the ARF6-based pathway is closely associated with immune evasion of KPC cells. Expression of ARF6 pathway components statistically correlated with poor patient outcomes. Thus, the cooperation among eIF4A/4E-dependent mRNA translation and MVP has emerged as a link by which pancreatic driver mutations may promote tumor cell motility, PD-L1 dynamics, and immune evasion, via empowering the ARF6-based pathway and its activation by external ligands.
Keywords: ARF6; PD-L1; mRNA translation; mevalonate pathway; pancreatic driver oncogenes.
Copyright © 2019 the Author(s). Published by PNAS.
Conflict of interest statement
Conflict of interest statement: H.S., S. Hashimoto, and A.H. are inventors on the patent application PCT/JP2019/10925.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

