Virus lasers for biological detection
- PMID: 31399594
- PMCID: PMC6689004
- DOI: 10.1038/s41467-019-11604-z
Virus lasers for biological detection
Abstract
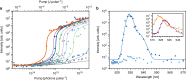
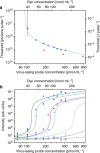
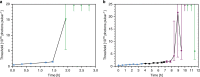
The selective amplification of DNA in the polymerase chain reaction is used to exponentially increase the signal in molecular diagnostics for nucleic acids, but there are no analogous techniques for signal enhancement in clinical tests for proteins or cells. Instead, the signal from affinity-based measurements of these biomolecules depends linearly on the probe concentration. Substituting antibody-based probes tagged for fluorescent quantification with lasing detection probes would create a new platform for biomarker quantification based on optical rather than enzymatic amplification. Here, we construct a virus laser which bridges synthetic biology and laser physics, and demonstrate virus-lasing probes for biosensing. Our virus-lasing probes display an unprecedented > 10,000 times increase in signal from only a 50% increase in probe concentration, using fluorimeter-compatible optics, and can detect biomolecules at sub-100 fmol mL-1 concentrations.
Conflict of interest statement
Research was partially funded by UCL Business, who have a financial interest in the commercial success of virus lasers. J.E.H., J.M.W. and G.A. are co-authors on a patent application (WO 2013/093499) and J.E.H., G.M., P.A.D. and G.A. are co-authors on a second patent application whose respective values could increase if the methods and ideas described in this paper find widespread application.
Figures


References
-
- Coons AH, Creech HJ, Jones RN. Immunological properties of an antibody containing a fluorescent group. P. Soc. Exp. Biol. Med. 1941;47:200–202. doi: 10.3181/00379727-47-13084P. - DOI
-
- Sorokin PP, Lankard JR. Stimulated emission observed from an organic dye, chloro-aluminum phthalocyanine. IBM J. Res. Dev. 1966;10:162–163. doi: 10.1147/rd.102.0162. - DOI
-
- Sorokin PP, Lankard JR. Flashlamp excitation of organic dye lasers: a short communication. IBM J. Res. Dev. 1967;11:148–148. doi: 10.1147/rd.112.0148. - DOI
-
- Peterson OG, Webb JP, McColgin WC, Eberly JH. Organic dye laser threshold. J. Appl. Phys. 1971;42:1917–1928. doi: 10.1063/1.1660468. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

