Extracellular vesicles isolated from porcine seminal plasma exhibit different tetraspanin expression profiles
- PMID: 31399634
- PMCID: PMC6689046
- DOI: 10.1038/s41598-019-48095-3
Extracellular vesicles isolated from porcine seminal plasma exhibit different tetraspanin expression profiles
Abstract
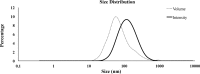
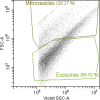
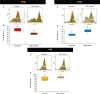
Seminal extracellular vesicles (EVs) include exosomes (ø 40-120 nm) and microvesicles (MVs, ø 120-1000 nm), which would be involved in multiple functional reproductive roles. The study aimed to establish which EV subtypes are present in pig semen, using a high-resolution flow cytometer to explore differences in their tetraspanin expression profile. The EVs were isolated from 12 pig ejaculates using serial ultracentrifugation and characterized by dynamic light scattering and electron microscopy for size and morphology as well as for tetraspanin expression using flow cytometry with Carboxyfluorescein succinimidyl ester (CFSE) and antibodies against CD9, CD63 and CD81. Pig semen contained a heterogeneous EV-population regarding size and morphology. Flow cytometric analysis demonstrated that the proportion of EVs expressing CD63 and CD9 was higher in MVs (P < 0.001 and P < 0.05, respectively) than in exosomes, while the opposite was true for CD81; higher (P < 0.001) in exosomes than in MVs. In conclusion, (1) the new generation of flow cytometers are able to accurately identify EVs and to gate them in two size-different populations named exosomes and MVs. (2) Tetraspanins CD9, CD63 and CD81 are present in both seminal EVs, albeit with exosomes and MVs differing in expression profiles, suggesting dissimilar cargo and binding affinity.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

