Amphetamines signal through intracellular TAAR1 receptors coupled to Gα13 and GαS in discrete subcellular domains
- PMID: 31399635
- PMCID: PMC7038576
- DOI: 10.1038/s41380-019-0469-2
Amphetamines signal through intracellular TAAR1 receptors coupled to Gα13 and GαS in discrete subcellular domains
Abstract
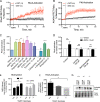
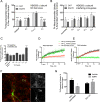
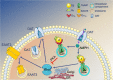
The extensive use of amphetamines to treat attention deficit hyperactivity disorders in children provides a compelling rationale for understanding the mechanisms of action of amphetamines and amphetamine-related drugs. We have previously shown that acute amphetamine (AMPH) regulates the trafficking of both dopamine and glutamate transporters in dopamine neurons by increasing activation of the small GTPase RhoA and of protein kinase A. Here we demonstrate that these downstream signaling events depend upon the direct activation of a trace amine-associated receptor, TAAR1, an intracellular G-protein coupled receptor (GPCR) that can be activated by amphetamines, trace amines, and biogenic amine metabolites. Using cell lines and mouse lines in which TAAR1 expression has been disrupted, we demonstrate that TAAR1 mediates the effects of AMPH on both RhoA and cAMP signaling. Inhibition of different Gα signaling pathways in cell lines and in vivo using small cell-permeable peptides confirms that the endogenous intracellular TAAR1 couples to G13 and to GS α-subunits to increase RhoA and PKA activity, respectively. Results from experiments with RhoA- and PKA-FRET sensors targeted to different subcellular compartments indicate that AMPH-elicited PKA activation occurs throughout the cell, whereas G13-mediated RhoA activation is concentrated near the endoplasmic reticulum. These observations define TAAR1 as an obligate intracellular target for amphetamines in dopamine neurons and support a model in which distinct pools of TAAR1 mediate the activation of signaling pathways in different compartments to regulate excitatory and dopaminergic neurotransmission.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



References
-
- Kahlig KM, Lute BJ, Wei Y, Loland CJ, Gether U, Javitch JA, et al. Regulation of dopamine transporter trafficking by intracellular amphetamine. Mol Pharmacol. 2006;70:542–8. - PubMed
-
- Bunzow JR, Sonders MS, Arttamangkul S, Harrison LM, Zhang G, Quigley DI, et al. Amphetamine, 3,4-methylenedioxymethamphetamine, lysergic acid diethylamide, and metabolites of the catecholamine neurotransmitters are agonists of a rat trace amine receptor. Mol Pharm. 2001;60:1181–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

