Personalizing prostate cancer diagnosis with multivariate risk prediction tools: how should prostate MRI be incorporated?
- PMID: 31399825
- PMCID: PMC7064475
- DOI: 10.1007/s00345-019-02899-0
Personalizing prostate cancer diagnosis with multivariate risk prediction tools: how should prostate MRI be incorporated?
Abstract
Risk-based patient selection for systematic biopsy in prostate cancer diagnosis has been adopted in daily clinical practice, either by clinical judgment and PSA testing, or using multivariate risk prediction tools. The use of multivariable risk prediction tools can significantly reduce unnecessary systematic biopsies, without compromising the detection of clinically significant disease. Increasingly multi-parametric magnetic resonance imaging (MRI) is performed, not only in men with a persistent suspicion of prostate cancer after prior negative systematic biopsy, but also at initial screening before the first biopsy. The combination of MRI and multivariate risk prediction tools could potentially enhance prostate cancer diagnosis using multivariate MRI incorporated risk-based models to decide on the need for prostate MRI, but also using MRI results to adjusted risk-based models, and to guide MRI-directed biopsies. In this review, we discuss the diagnostic work-up for clinically significant prostate cancer, where the combination of MRI and multivariate risk prediction tools is integrated, and how together they can contribute to personalized diagnosis.
Keywords: Biopsy; Magnetic resonance imaging (MRI); Multivariate risk prediction; Nomogram; Prostate cancer; Risk calculator; Risk stratification.
Conflict of interest statement
The authors have no potential conflicts of interest to declare.
Figures



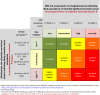
References
-
- European Association of Urology (EAU) (2019) Guidelines on prostate cancer. http://uroweb.org/guideline/prostate-cancer/. Accessed Apr 2019
-
- Prostate cancer: diagnosis and management (update) (CG175) (2019) National institute for health and care excellence (NICE) Web site. https://www.nice.org.uk/guidance/cg175. Accessed April 2019
-
- National Comprehensive Cancer Network (NCCN) (2018) Guidelines on prostate cancer: 2018 update. https://www.nccn.org/professionals/physician_gls/default.aspx. Accessed Apr 2019
-
- Kasivisvanathan V, Rannikko AS, Borghi M, Panebianco V, Mynderse LA, Vaarala MH, Briganti A, Budäus L, Hellawell G, Hindley RG, Roobol MJ, Eggener S, Ghei M, Villers A, Bladou F, Villeirs GM, Virdi J, Boxler S, Robert G, Singh PB, Venderink W, Hadaschik BA, Ruffion A, Hu JC, Margolis D, Crouzet S, Klotz L, Taneja SS, Pinto P, Gill I, Allen C, Giganti F, Freeman A, Morris S, Punwani S, Williams NR, Brew-Graves C, Deeks J, Takwoingi Y, Emberton M, Moore CM. MRI-targeted or standard biopsy for prostate-cancer diagnosis. N Engl J Med. 2018;378(19):1767–1777. doi: 10.1056/NEJMoa1801993. - DOI - PMC - PubMed
-
- Porpiglia F, Manfredi M, Mele F, Cossu M, Bollito E, Veltri A, Cirillo S, Regge D, Faletti R, Passera R, Fiori C, De Luca S. Diagnostic pathway with multiparametric magnetic resonance imaging versus standard pathway: results from a randomized prospective study in biopsy-naive patients with suspected prostate cancer. Eur Urol. 2017;72(2):282–288. doi: 10.1016/j.eururo.2016.08.041. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

