Malaria Molecular Epidemiology: An Evolutionary Genetics Perspective
- PMID: 31400095
- PMCID: PMC6690375
- DOI: 10.1128/microbiolspec.AME-0010-2019
Malaria Molecular Epidemiology: An Evolutionary Genetics Perspective
Abstract
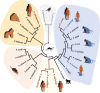
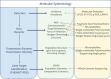
Malaria is a vector-borne disease that involves multiple parasite species in a variety of ecological settings. However, the parasite species causing the disease, the prevalence of subclinical infections, the emergence of drug resistance, the scale-up of interventions, and the ecological factors affecting malaria transmission, among others, are aspects that vary across areas where malaria is endemic. Such complexities have propelled the study of parasite genetic diversity patterns in the context of epidemiologic investigations. Importantly, molecular studies indicate that the time and spatial distribution of malaria cases reflect epidemiologic processes that cannot be fully understood without characterizing the evolutionary forces shaping parasite population genetic patterns. Although broad in scope, this review in the Microbiology Spectrum Curated Collection: Advances in Molecular Epidemiology highlights the need for understanding population genetic concepts when interpreting parasite molecular data. First, we discuss malaria complexity in terms of the parasite species involved. Second, we describe how molecular data are changing our understanding of malaria incidence and infectiousness. Third, we compare different approaches to generate parasite genetic information in the context of epidemiologically relevant questions related to malaria control. Finally, we describe a few Plasmodium genomic studies as evidence of how these approaches will provide new insights into the malaria disease dynamics. *This article is part of a curated collection.
Figures

References
-
- World Health Organization. 2018. World Malaria Report 2018. World Health Organization, Geneva, Switzerland. https://www.who.int/malaria/publications/world-malaria-report-2018/en/.
-
- Coatney RG, Collins WE, Warren M, Contacos PG. 1971. The Primate Malaria. US Government Printing Office, Washington, DC.
-
- Muehlenbein MP, Pacheco MA, Taylor JE, Prall SP, Ambu L, Nathan S, Alsisto S, Ramirez D, Escalante AA. 2015. Accelerated diversification of nonhuman primate malarias in Southeast Asia: adaptive radiation or geographic speciation? Mol Biol Evol 32:422–439. 10.1093/molbev/msu310. [PubMed] - DOI - PMC - PubMed
-
- Otto TD, Rayner JC, Böhme U, Pain A, Spottiswoode N, Sanders M, Quail M, Ollomo B, Renaud F, Thomas AW, Prugnolle F, Conway DJ, Newbold C, Berriman M. 2014. Genome sequencing of chimpanzee malaria parasites reveals possible pathways of adaptation to human hosts. Nat Commun 5:4754. 10.1038/ncomms5754. [PubMed] - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

