A deep learning genome-mining strategy for biosynthetic gene cluster prediction
- PMID: 31400112
- PMCID: PMC6765103
- DOI: 10.1093/nar/gkz654
A deep learning genome-mining strategy for biosynthetic gene cluster prediction
Abstract
Natural products represent a rich reservoir of small molecule drug candidates utilized as antimicrobial drugs, anticancer therapies, and immunomodulatory agents. These molecules are microbial secondary metabolites synthesized by co-localized genes termed Biosynthetic Gene Clusters (BGCs). The increase in full microbial genomes and similar resources has led to development of BGC prediction algorithms, although their precision and ability to identify novel BGC classes could be improved. Here we present a deep learning strategy (DeepBGC) that offers reduced false positive rates in BGC identification and an improved ability to extrapolate and identify novel BGC classes compared to existing machine-learning tools. We supplemented this with random forest classifiers that accurately predicted BGC product classes and potential chemical activity. Application of DeepBGC to bacterial genomes uncovered previously undetectable putative BGCs that may code for natural products with novel biologic activities. The improved accuracy and classification ability of DeepBGC represents a major addition to in-silico BGC identification.
© The Author(s) 2019. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures


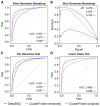
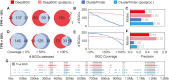

References
-
- Pendleton J.N., Gorman S.P., Gilmore B.F.. Clinical relevance of the ESKAPE pathogens. Expert Rev. Anti. Infect. Ther. 2013; 11:297–308. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

