Scalability of cancer SurvivorLink™: A cluster randomized trial among pediatric cancer clinics
- PMID: 31400518
- PMCID: PMC7928166
- DOI: 10.1016/j.cct.2019.105819
Scalability of cancer SurvivorLink™: A cluster randomized trial among pediatric cancer clinics
Abstract
Background: Children diagnosed with cancer are living longer and the survivor population is growing. However, most survivors develop late effects of radiation and chemotherapy shortly to years after completion of therapy, and the receipt of follow-up visits that are recommended by the Children's Oncology Group (COG) is suboptimal nationally.
Aims: The aims of this study are to: 1) evaluate the impact of a patient-controlled electronic personal health record (ePHR) and system (SurvivorLink) on care visit attendance, risk-based surveillance, and other secondary outcomes (i.e., patient activation, quality of life (QOL)); 2) measure the use, acceptability, and perceived usefulness of, and satisfaction with SurvivorLink; and 3) assess facilitators and barriers to implementation.
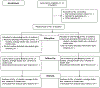
Methods: This hybrid effectiveness-implementation, clustered randomized control trial (RCT) evaluates the effect of SurvivorLink among pediatric cancer survivors and their parents on receipt of follow-up cancer care. We will recruit 20 pediatric survivor clinics with half receiving the intervention and half acting as a waitlist control. Parents of survivors and survivors will complete baseline, 3 and 12 month surveys that assess SurvivorLink use, patient self-efficacy, and intentions to return for follow-up. We will use mixed methods and multi-informant assessment to assess implementation outcomes (i.e., acceptability, feasibility, appropriateness).
Discussion: New approaches are needed to facilitate the receipt of long-term follow-up care among pediatric cancer survivors. This study will assess whether SurvivorLink is effective in increasing receipt of follow-up cancer care. Moreover, it will explore the influences of context and other moderators of clinical practice change in pediatric cancer survivorship.
Keywords: Cancer survivor; Child; Health behavior; Health education; Medical records; Surveys and questionnaire.
Copyright © 2019. Published by Elsevier Inc.
Conflict of interest statement
Competing interests
The authors declare that they have no competing interests.
Figures
Similar articles
-
Meaningful Use of an Electronic Personal Health Record (ePHR) among Pediatric Cancer Survivors.Appl Clin Inform. 2017 Mar 15;8(1):250-264. doi: 10.4338/ACI-2016-11-RA-0189. Appl Clin Inform. 2017. PMID: 28293684 Free PMC article.
-
Predictors of successful use of a web-based healthcare document storage and sharing system for pediatric cancer survivors: Cancer SurvivorLink™.J Cancer Surviv. 2014 Sep;8(3):355-63. doi: 10.1007/s11764-014-0346-6. Epub 2014 Feb 18. J Cancer Surviv. 2014. PMID: 24535124
-
The Head and Neck Survivorship Tool (HN-STAR) Trial (WF-1805CD): A protocol for a cluster-randomized, hybrid effectiveness-implementation, pragmatic trial to improve the follow-up care of head and neck cancer survivors.Contemp Clin Trials. 2021 Aug;107:106448. doi: 10.1016/j.cct.2021.106448. Epub 2021 May 21. Contemp Clin Trials. 2021. PMID: 34023515 Free PMC article.
-
Cancer survivorship practices, services, and delivery: a report from the Children's Oncology Group (COG) nursing discipline, adolescent/young adult, and late effects committees.J Cancer Surviv. 2011 Dec;5(4):345-57. doi: 10.1007/s11764-011-0192-8. Epub 2011 Sep 6. J Cancer Surviv. 2011. PMID: 21894490 Free PMC article. Review.
-
Late Effects and Long-Term Follow-Up after Cancer in Childhood.Oncol Res Treat. 2017;40(12):746-750. doi: 10.1159/000484936. Epub 2017 Nov 29. Oncol Res Treat. 2017. PMID: 29183026 Review.
Cited by
-
Understanding caregivers' decision to vaccinate childhood cancer survivors against COVID-19.Cancer Med. 2023 Dec;12(23):21354-21363. doi: 10.1002/cam4.6675. Epub 2023 Nov 8. Cancer Med. 2023. PMID: 37937725 Free PMC article.
-
Clinic reported facilitators and barriers to pediatric cancer survivor care delivery among survivorship clinics: A fishbone analysis.Pediatr Blood Cancer. 2023 Aug;70(8):e30480. doi: 10.1002/pbc.30480. Epub 2023 Jun 3. Pediatr Blood Cancer. 2023. PMID: 37269530 Free PMC article.
-
Characterization of Patient Activation among Childhood Cancer Survivors in the St. Jude Lifetime Cohort Study (SJLIFE).Cancers (Basel). 2024 Sep 21;16(18):3220. doi: 10.3390/cancers16183220. Cancers (Basel). 2024. PMID: 39335191 Free PMC article.
References
-
- Howlader N, Noone A, Krapcho M, Garshell J, Neyman N, Altekruse S. SEER Cancer Statistics Review, 1975-2014. Bethesda, MD; 2016.
-
- Oeffinger KC, Hudson MM. Long-term complications following childhood and adolescent cancer: foundations for providing risk-based health care for survivors. CA Cancer J Clin. 2004;54(4):208–36. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical