Longer-term open-label study of adjunctive riluzole in treatment-resistant depression
- PMID: 31400624
- PMCID: PMC6710149
- DOI: 10.1016/j.jad.2019.06.065
Longer-term open-label study of adjunctive riluzole in treatment-resistant depression
Abstract
Background: While riluzole has been investigated for the treatment of depression, little is known about its longer-term efficacy and optimal treatment duration in treatment-resistant depression (TRD). The objective of this study is to characterize the longer-term outcome of adjunctive riluzole therapy for TRD in an open-label extension of an 8-week acute treatment trial.
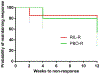
Methods: The data from 66 patients with TRD who received adjunctive riluzole in a 12-week open-label extension phase were analyzed. Response rates (⩾50% reduction in the Mongomery-Asberg Depression Rating Scale [MADRS] score), relapse rates (a MADRS score of ⩾22 in patients who had previously achieved response), and adverse events were examined in patients who had achieved response at the end of the acute phase and those who had not.
Results: Among acute phase responders, the maintained response rate was 66.7% (8/12) and the relapse rate was 8.3% (1/12). In acute phase non-responders, the response rate was 24.1% (13/54). The most commonly reported adverse event was fatigue (9.1%). Three cases were considered serious adverse events; vomiting (n = 1), shortness of breath (n = 1), and aborted suicide attempt (n = 1).
Limitations: This longer-term study was open-label and uncontrolled. The sample size was relatively small.
Conclusions: Longer-term adjunctive riluzole appears relatively well tolerated and beneficial for maintaining previous response. Additionally, approximately one fourth of patients who did not respond to 8-week antidepressant treatment might respond if treated with riluzole for 12 weeks. Those findings warrant further investigation because adjunctive riluzole could represent an option for treatment of depression when standard antidepressants have failed.
Keywords: Longer-term; Relapse; Response; Riluzole; Tolerability; Treatment-resistant depression.
Copyright © 2019. Published by Elsevier B.V.
Conflict of interest statement
Declarations of interest:
Dr. Sakurai has received manuscript or speaker’s honoraria from Dainippon Sumitomo, Eli Lilly, Meiji-Seika Pharma, Otsuka Pharmaceutical, Tanabe Mitsubishi Pharma, and Yoshitomi Yakuhin within the past three years. He also receives support from the Japanese Society of Clinical Neuropsychopharmacology. Dr. Chang had stock of Abbott and Shire Pharmaceuticals and received support from an NIDDK career development award. Dr. Wilkinson reports research funding and consulting fees from Janssen. He also receives support from the Agency for Healthcare Research and Quality (K12HS023000) and from the Brain and Behavioral Research Foundation (formerly NARSAD), the Robert E. Leet and Clara Guthrie Patterson Trust, and the American Foundation for Suicide Prevention. Dr. Mathew is supported by the use of facilities and resources at the Michael E. Debakey VA Medical Center, Houston, Tx. He has served as a consultant to Alkermes, Bracket, Clexio, Janssen, Perception, and Sage Therapeutics. He has received research support from Biohaven, NeuroRx, and Vistagen. Dr. Mischoulon has received research support from Nordic Naturals. He has provided unpaid consulting for Pharmavite LLC and Gnosis USA, Inc. He has received honoraria for speaking from the Massachusetts General Hospital Psychiatry Academy, Blackmores, and PeerPoint Medical Education Institute, LLC. He has received royalties from Lippincott Williams & Wilkins for published book “Natural Medications for Psychiatric Disorders: Considering the Alternatives.” Dr. Fava reports 3-year disclosures as below: all lifetime disclosures can be view on line at:
Figures
References
-
- Banasr M, Chowdhury GM, Terwilliger R, Newton SS, Duman RS, Behar KL, Sanacora G, 2010. Glial pathology in an animal model of depression: reversal of stress-induced cellular, metabolic and behavioral deficits by the glutamate-modulating drug riluzole. Mol Psychiatry. 15, 501–511. 10.1038/mp.2008.106 - DOI - PMC - PubMed
-
- Brennan BP, Hudson JI, Jensen JE, McCarthy J, Roberts JL, Prescot AP, Cohen BM, Pope HG Jr., Renshaw PF, Ongür D, 2010. Rapid enhancement of glutamatergic neurotransmission in bipolar depression following treatment with riluzole. Neuropsychopharmacology. 35, 834–846. 10.1038/npp.2009.191 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical