A conserved histidine in Group-1 influenza subtype hemagglutinin proteins is essential for membrane fusion activity
- PMID: 31401467
- PMCID: PMC6733629
- DOI: 10.1016/j.virol.2019.08.005
A conserved histidine in Group-1 influenza subtype hemagglutinin proteins is essential for membrane fusion activity
Abstract
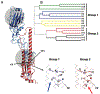
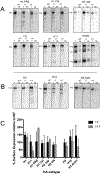
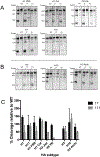
Influenza A viruses enter host cells through the endocytic pathway, where acidification triggers conformational changes of the viral hemagglutinin (HA) to drive membrane fusion. During this process, the HA fusion peptide is extruded from its buried position in the neutral pH structure and targeted to the endosomal membrane. Conserved ionizable residues near the fusion peptide may play a role in initiating these structural rearrangements. We targeted highly conserved histidine residues in this region, at HA1 position 17 of Group-2 HA subtypes and HA2 position 111 of Group-1 HA subtypes, to determine their role in fusion activity. WT and mutant HA proteins representing several subtypes were expressed and characterized, revealing that His 111 is essential for HA functional activity of Group-1 subtypes, supporting continued efforts to target this region of the HA structure for vaccination strategies and the design of antiviral compounds.
Keywords: Fusion; Hemagglutinin; Influenza; Virus entry.
Copyright © 2019 Elsevier Inc. All rights reserved.
Figures



References
-
- Bizebard T, Gigant B, Rigolet P, Rasmussen B, Diat O, Bosecke P, Wharton SA, Skehel JJ, Knossow M, 1995. Structure of influenza virus haemagglutinin complexed with a neutralizing antibody. Nature 376, 92–94. - PubMed
-
- Bodian DL, Yamasaki RB, Buswell RL, Stearns JF, White JM, Kuntz ID, 1993. Inhibition of the fusion-inducing conformational change of influenza hemagglutinin by benzoquinones and hydroquinones. Biochemistry 32, 2967–2978. - PubMed
-
- Bullough PA, Hughson FM, Skehel JJ, Wiley DC, 1994. Structure of influenza haemagglutinin at the pH of membrane fusion. Nature 371, 37–43. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

