AK002, a Humanized Sialic Acid-Binding Immunoglobulin-Like Lectin-8 Antibody that Induces Antibody-Dependent Cell-Mediated Cytotoxicity against Human Eosinophils and Inhibits Mast Cell-Mediated Anaphylaxis in Mice
- PMID: 31401630
- PMCID: PMC6878738
- DOI: 10.1159/000501637
AK002, a Humanized Sialic Acid-Binding Immunoglobulin-Like Lectin-8 Antibody that Induces Antibody-Dependent Cell-Mediated Cytotoxicity against Human Eosinophils and Inhibits Mast Cell-Mediated Anaphylaxis in Mice
Abstract
Introduction: Pathologic accumulation and activation of mast cells and eosinophils are implicated in allergic and inflammatory diseases. Sialic acid-binding immunoglobulin-like lectin (Siglec)-8 is an inhibitory receptor selectively expressed on mast cells, eosinophils and, at a lower extent, basophils. When engaged with an antibody, Siglec-8 can induce apoptosis of activated eosinophils and inhibit mast cell activation. AK002 is a humanized, non-fucosylated IgG1 anti-Siglec-8 antibody undergoing clinical investigation for treatment of allergic, inflammatory, and proliferative diseases. Here we examine the human tissue selectivity of AK002 and evaluate the in vitro, ex vivo, and in vivo activity of AK002 on eosinophils and mast cells.
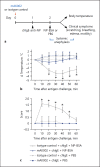
Methods: The affinity of AK002 for Siglec-8 and CD16 was determined by biolayer interferometry. Ex vivo activity of AK002 on human eosinophils from blood and dissociated human tissue was tested in apoptosis and antibody-dependent cell-mediated cytotoxicity (ADCC) assays. The in vivo activity of a murine precursor of AK002 (mAK002) was tested in a passive systemic anaphylaxis (PSA) humanized mouse model.
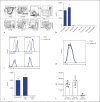
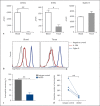
Results: AK002 bound selectively to mast cells, eosinophils and, at a lower level, to basophils in human blood and tissue and not to other cell types examined. AK002 induced apoptosis of interleukin-5-activated blood eosinophils and demonstrated potent ADCC activity against blood eosinophils in the presence of natural killer cells. AK002 also significantly reduced eosinophils in dissociated human lung tissue. Furthermore, mAK002 prevented PSA in humanized mice through mast cell inhibition.
Conclusion: AK002 selectively evokes potent apoptotic and ADCC activity against eosinophils and prevents systemic anaphylaxis through mast cell inhibition.
Keywords: AK002; Anaphylaxis; Antibody-dependent cell-mediated cytotoxicity; Eosinophils; Mast cells; Sialic acid-binding immunoglobulin-like lectin-8.
© 2019 The Author(s)Published by S. Karger AG, Basel.
Conflict of interest statement
Drs. Bradford A. Youngblood, Rustom Falahati, Christopher Bebbington, and Nenad Tomasevic, and Ms. Emily C. Brock and Jessica Bright and Mr. John Leung and Jason Williams are or were employees and have ownership interest in Allakos Inc., outside the submitted work; Drs. Leonard D. Shultz, Dale L. Greiner, and Michael A. Brehm report personal fees from Allakos, during the conduct of the study. Dr. Leonard D. Shultz also reports personal fees from Allakos, outside the submitted work; Drs. Dale L. Greiner and Michael A. Brehm also received grant support and were consultants for The Jackson Laboratory. Dr. Paul J. Bryce has no conflicts of interest to disclose.
Figures



References
-
- Kikly KK, Bochner BS, Freeman SD, Tan KB, Gallagher KT, D'alessio KJ, et al. Identification of SAF-2, a novel siglec expressed on eosinophils, mast cells, and basophils. J Allergy Clin Immunol. 2000 Jun;105((6 Pt 1)):1093–100. - PubMed
-
- Floyd H, Ni J, Cornish AL, Zeng Z, Liu D, Carter KC, et al. Siglec-8. A novel eosinophil-specific member of the immunoglobulin superfamily. J Biol Chem. 2000 Jan;275((2)):861–6. - PubMed
-
- Yokoi H, Choi OH, Hubbard W, Lee HS, Canning BJ, Lee HH, et al. Inhibition of FcepsilonRI-dependent mediator release and calcium flux from human mast cells by sialic acid-binding immunoglobulin-like lectin 8 engagement. J Allergy Clin Immunol. 2008 Feb;121((2)):499–505.e1. - PubMed
-
- Nutku E, Aizawa H, Hudson SA, Bochner BS. Ligation of Siglec-8: a selective mechanism for induction of human eosinophil apoptosis. Blood. 2003 Jun;101((12)):5014–20. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

