Dose-response and transmission: the nexus between reservoir hosts, environment and recipient hosts
- PMID: 31401955
- PMCID: PMC6711301
- DOI: 10.1098/rstb.2019.0016
Dose-response and transmission: the nexus between reservoir hosts, environment and recipient hosts
Abstract
Dose is the nexus between exposure and all upstream processes that determine pathogen pressure, and is thereby an important element underlying disease dynamics. Understanding the relationship between dose and disease is particularly important in the context of spillover, where nonlinearities in the dose-response could determine the likelihood of transmission. There is a need to explore dose-response models for directly transmitted and zoonotic pathogens, and how these interactions integrate within-host factors to consider, for example, heterogeneity in host susceptibility and dose-dependent antagonism. Here, we review the dose-response literature and discuss the unique role dose-response models have to play in understanding and predicting spillover events. We present a re-analysis of dose-response experiments for two important zoonotic pathogens (Middle East respiratory syndrome coronavirus and Nipah virus), to exemplify potential difficulties in differentiating between appropriate models with small exposure experiment datasets. We also discuss the data requirements needed for robust selection between dose-response models. We then suggest how these processes could be modelled to gain more realistic predictions of zoonotic transmission outcomes and highlight the exciting opportunities that could arise with increased collaboration between the virology and epidemiology disciplines. This article is part of the theme issue 'Dynamic and integrative approaches to understanding pathogen spillover'.
Keywords: infection; infectious disease; modelling; nonlinearities; spillover; virus.
Conflict of interest statement
We declare we have no competing interests.
Figures
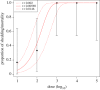
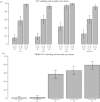
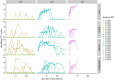
References
-
- Conlan AJ, Line JE, Hiett K, Coward C, Van Diemen PM, Stevens MP, Jones MA, Gog JR, Maskell DJ. 2011. Transmission and dose–response experiments for social animals: a reappraisal of the colonization biology of Campylobacter jejuni in chickens. J. R. Soc. Interface 8, 1720–1735. (10.1098/rsif.2011.0125) - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

