Molecular Strategies of Meiotic Cheating by Selfish Centromeres
- PMID: 31402175
- PMCID: PMC6731994
- DOI: 10.1016/j.cell.2019.07.001
Molecular Strategies of Meiotic Cheating by Selfish Centromeres
Abstract
Asymmetric division in female meiosis creates selective pressure favoring selfish centromeres that bias their transmission to the egg. This centromere drive can explain the paradoxical rapid evolution of both centromere DNA and centromere-binding proteins despite conserved centromere function. Here, we define a molecular pathway linking expanded centromeres to histone phosphorylation and recruitment of microtubule destabilizing factors, leading to detachment of selfish centromeres from spindle microtubules that would direct them to the polar body. Exploiting centromere divergence between species, we show that selfish centromeres in two hybrid mouse models use the same molecular pathway but modulate it differently to enrich destabilizing factors. Our results indicate that increasing microtubule destabilizing activity is a general strategy for drive in both models, but centromeres have evolved distinct mechanisms to increase that activity. Furthermore, we show that drive depends on slowing meiotic progression, suggesting that selfish centromeres can be suppressed by regulating meiotic timing.
Keywords: centromere; chromosome segregation; meiosis; meiotic drive; mouse; oocyte.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
DECLARATION OF INTERESTS
The authors declare no competing interests.
Figures


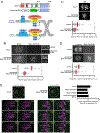

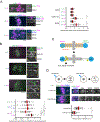

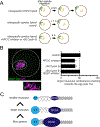
Comment in
-
Meiosis: How Gambling Chromosomes Beat the Rules.Curr Biol. 2019 Dec 2;29(23):R1247-R1248. doi: 10.1016/j.cub.2019.10.056. Curr Biol. 2019. PMID: 31794760
References
-
- Almonacid M, Terret M-E, and Verlhac M-H (2014). Actin-based spindle positioning: new insights from female gametes. J. Cell Sci. 127, 477–483. - PubMed
-
- Azoury J, Lee KW, Georget V, Hikal P, and Verlhac M-H (2011). Symmetry breaking in mouse oocytes requires transient F-actin meshwork destabilization. Development 138, 2903–2908. - PubMed
-
- Bint SM, Ogilvie CM, Flinter FA, Khalaf Y, and Scriven PN (2011). Meiotic segregation of Robertsonian translocations ascertained in cleavage-stage embryos--implications for preimplantation genetic diagnosis. Hum. Reprod 26, 1575–1584. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

