Leigh Syndrome Mouse Model Can Be Rescued by Interventions that Normalize Brain Hyperoxia, but Not HIF Activation
- PMID: 31402314
- PMCID: PMC6903907
- DOI: 10.1016/j.cmet.2019.07.006
Leigh Syndrome Mouse Model Can Be Rescued by Interventions that Normalize Brain Hyperoxia, but Not HIF Activation
Abstract
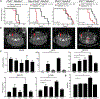
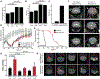
Leigh syndrome is a devastating mitochondrial disease for which there are no proven therapies. We previously showed that breathing chronic, continuous hypoxia can prevent and even reverse neurological disease in the Ndufs4 knockout (KO) mouse model of complex I (CI) deficiency and Leigh syndrome. Here, we show that genetic activation of the hypoxia-inducible factor transcriptional program via any of four different strategies is insufficient to rescue disease. Rather, we observe an age-dependent decline in whole-body oxygen consumption. These mice exhibit brain tissue hyperoxia, which is normalized by hypoxic breathing. Alternative experimental strategies to reduce oxygen delivery, including breathing carbon monoxide (600 ppm in air) or severe anemia, can reverse neurological disease. Therefore, unused oxygen is the most likely culprit in the pathology of this disease. While pharmacologic activation of the hypoxia response is unlikely to alleviate disease in vivo, interventions that safely normalize brain tissue hyperoxia may hold therapeutic potential.
Keywords: Leigh syndrome; anemia; carbon monoxide; hemoglobin; hyperoxia; hypoxia; mitochondria; oxygen; therapy.
Copyright © 2019. Published by Elsevier Inc.
Figures


Comment in
-
Breathe: Your Mitochondria Will Do the Rest… If They Are Healthy!Cell Metab. 2019 Oct 1;30(4):628-629. doi: 10.1016/j.cmet.2019.09.004. Cell Metab. 2019. PMID: 31577932
Similar articles
-
Hypoxia ameliorates brain hyperoxia and NAD+ deficiency in a murine model of Leigh syndrome.Mol Genet Metab. 2021 May;133(1):83-93. doi: 10.1016/j.ymgme.2021.03.005. Epub 2021 Mar 11. Mol Genet Metab. 2021. PMID: 33752971 Free PMC article.
-
Hypoxia treatment reverses neurodegenerative disease in a mouse model of Leigh syndrome.Proc Natl Acad Sci U S A. 2017 May 23;114(21):E4241-E4250. doi: 10.1073/pnas.1621511114. Epub 2017 May 8. Proc Natl Acad Sci U S A. 2017. PMID: 28483998 Free PMC article.
-
Decreased VEGF expression and microvascular density, but increased HIF-1 and 2α accumulation and EPO expression in chronic moderate hyperoxia in the mouse brain.Brain Res. 2012 Aug 30;1471:46-55. doi: 10.1016/j.brainres.2012.06.055. Epub 2012 Jul 20. Brain Res. 2012. PMID: 22820296 Free PMC article.
-
Keeping the engine primed: HIF factors as key regulators of cardiac metabolism and angiogenesis during ischemia.J Mol Med (Berl). 2007 Dec;85(12):1309-15. doi: 10.1007/s00109-007-0279-x. Epub 2007 Nov 20. J Mol Med (Berl). 2007. PMID: 18026917 Review.
-
Hypoxia and the HIF system in kidney disease.J Mol Med (Berl). 2007 Dec;85(12):1325-30. doi: 10.1007/s00109-007-0278-y. Epub 2007 Nov 20. J Mol Med (Berl). 2007. PMID: 18026918 Review.
Cited by
-
Generation of Reactive Oxygen Species by Mitochondria.Antioxidants (Basel). 2021 Mar 9;10(3):415. doi: 10.3390/antiox10030415. Antioxidants (Basel). 2021. PMID: 33803273 Free PMC article. Review.
-
Mitochondrial lactate metabolism: history and implications for exercise and disease.J Physiol. 2021 Feb;599(3):863-888. doi: 10.1113/JP278930. Epub 2020 May 27. J Physiol. 2021. PMID: 32358865 Free PMC article. Review.
-
Small-molecule hypoxia therapy in mitochondrial disease.Cell. 2025 Mar 20;188(6):1462-1465. doi: 10.1016/j.cell.2025.02.019. Cell. 2025. PMID: 40118030
-
The new role of F1Fo ATP synthase in mitochondria-mediated neurodegeneration and neuroprotection.Exp Neurol. 2020 Oct;332:113400. doi: 10.1016/j.expneurol.2020.113400. Epub 2020 Jul 10. Exp Neurol. 2020. PMID: 32653453 Free PMC article. Review.
-
Defective function of α-ketoglutarate dehydrogenase exacerbates mitochondrial ATP deficits during complex I deficiency.Redox Biol. 2023 Nov;67:102932. doi: 10.1016/j.redox.2023.102932. Epub 2023 Oct 17. Redox Biol. 2023. PMID: 37883842 Free PMC article.
References
-
- Aragoné s J, Schneider M, Van Geyte K, Fraisl P, Dresselaers T, Mazzone M, Dirkx R, Zacchigna S, Lemieux H, Jeoung NH, et al. (2008). Deficiency or inhibition of oxygen sensor Phd1 induces hypoxia tolerance by reprogramming basal metabolism. Nat. Genet. 40, 170–180. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

