Serologic Markers of Previous Malaria Exposure and Functional Antibodies Inhibiting Parasite Growth Are Associated With Parasite Kinetics Following a Plasmodium falciparum Controlled Human Infection
- PMID: 31402382
- PMCID: PMC7286377
- DOI: 10.1093/cid/ciz740
Serologic Markers of Previous Malaria Exposure and Functional Antibodies Inhibiting Parasite Growth Are Associated With Parasite Kinetics Following a Plasmodium falciparum Controlled Human Infection
Erratum in
-
Erratum.Clin Infect Dis. 2020 Jan 2;70(2):359. doi: 10.1093/cid/ciz1048. Clin Infect Dis. 2020. PMID: 31750890 Free PMC article. No abstract available.
Abstract
Background: We assessed the impact of exposure to Plasmodium falciparum on parasite kinetics, clinical symptoms, and functional immunity after controlled human malaria infection (CHMI) in 2 cohorts with different levels of previous malarial exposure.
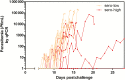
Methods: Nine adult males with high (sero-high) and 10 with low (sero-low) previous exposure received 3200 P. falciparum sporozoites (PfSPZ) of PfSPZ Challenge by direct venous inoculation and were followed for 35 days for parasitemia by thick blood smear (TBS) and quantitative polymerase chain reaction. Endpoints were time to parasitemia, adverse events, and immune responses.
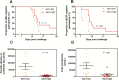
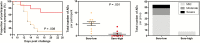
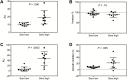
Results: Ten of 10 (100%) volunteers in the sero-low and 7 of 9 (77.8%) in the sero-high group developed parasitemia detected by TBS in the first 28 days (P = .125). The median time to parasitemia was significantly shorter in the sero-low group than the sero-high group (9 days [interquartile range {IQR} 7.5-11.0] vs 11.0 days [IQR 7.5-18.0], respectively; log-rank test, P = .005). Antibody recognition of sporozoites was significantly higher in the sero-high (median, 17.93 [IQR 12.95-24] arbitrary units [AU]) than the sero-low volunteers (median, 10.54 [IQR, 8.36-12.12] AU) (P = .006). Growth inhibitory activity was significantly higher in the sero-high (median, 21.8% [IQR, 8.15%-29.65%]) than in the sero-low group (median, 8.3% [IQR, 5.6%-10.23%]) (P = .025).
Conclusions: CHMI was safe and well tolerated in this population. Individuals with serological evidence of higher malaria exposure were able to better control infection and had higher parasite growth inhibitory activity.
Clinical trials registration: NCT03496454.
Keywords: clinical outcomes; controlled human malaria infection; functional antibodies; malaria exposure; parasite kinetics.
© The Author(s) 2019. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures

References
-
- Fowkes FJ, Boeuf P, Beeson JG. Immunity to malaria in an era of declining malaria transmission. Parasitology 2016; 143:139–53. - PubMed
-
- Hoffman SL, Oster CN, Plowe CV, et al. Naturally acquired antibodies to sporozoites do not prevent malaria: vaccine development implications. Science 1987; 237:639–42. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

